[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]The Chemistry of Art > 4. The Transition Metals > What is a Transition Element? >[/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Define the term transition element[/cs_text][cs_text]
- Definition: Elements that form at least one ion with a partially filled sub-shell of d electrons
- Lesser definitions:
- Transition elements are those that have partially filled d-orbitals
- Transition elements are those that have properties intermediate between s-block and p-block elements
Elements in the d-block that are not transition metals:
- Scandium: Its only ion (Sc3+) has no electrons in the d sub-shell
Scandium atom:[/cs_text][cs_text]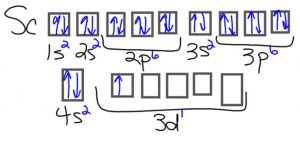 [/cs_text][cs_text]
[/cs_text][cs_text] [/cs_text][cs_text]
[/cs_text][cs_text]
- Zinc: Its only ion (Zn2+) has a completely filled d sub-shell
[/cs_text][cs_text]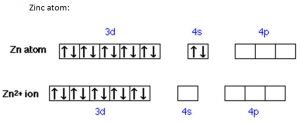 [/cs_text][cs_text]Elements with unique orbital filling patterns: (For a later dotpoint but whatever)
[/cs_text][cs_text]Elements with unique orbital filling patterns: (For a later dotpoint but whatever)
- Filling typically occurs according to Hund’s rule
- Electrons in each of the 5 3d-orbitals
- 4s orbital already filled due to relative energy compared to 3p
- Exceptions to this rule are Chromium and Copper
[/cs_text][cs_text] [/cs_text][cs_text]
[/cs_text][cs_text]
- More stable when all d- orbital are half filled
- Electron moves from already filled 4s shell to accommodate
[/cs_text][cs_text] [/cs_text][cs_text]
[/cs_text][cs_text]
- More stable when all d-orbtials are filled with pairs
- Electron moves from already filled 4s shell to accommodate
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]