Steps in writing equilibrium constant expression:
- The concentrations of the reacting species in the condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm.
- The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions.
- The equilibrium constant is a dimensionless quantity.
- In quoting a value for the equilibrium constant, you must specify the balanced equation and the temperature.
- If a reaction can be expressed as a sum of two or more reactions, the equilibrium constant for the overall reaction is given by the product of the equilibrium constants of the individual reactions.
To calculate for equilibrium concentrations:
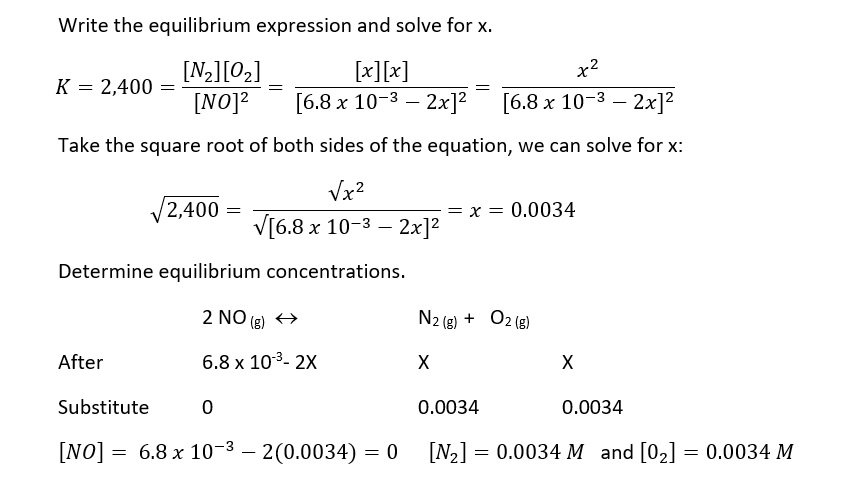
- Express the equilibrium concentrations of all species in terms of the initial concentrations and a single unknown x, which represents the change in concentration.
- Write the equilibrium constant expression in terms of the equilibrium concentrations. Knowing the value of the equilibrium constant, solve for x.
- Having solved for x, calculate the equilibrium concentrations of all species
Sample Problem:
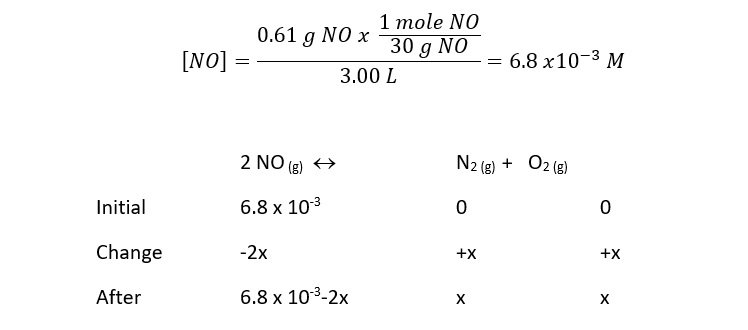
The reaction 2 NO (g) « N2 (g) + O2 (g) has a value of Keq= 2,400 at a temperature of 2,000 K. If 0.61 g of NO are put in a previously empty 3.00 L vessel, calculate the equilibrium concentrations of NO, N2, and O2.
Solution: Make a chart describing relationships in change. The initial concentration of the reactant can be determined by: