Le Châtelier’s principle states that if an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is partially offset as it tries to re-establish equilibrium. The word “stress” means a change in temperature, pressure, volume or concentration of reactants in an equilibrated system. Le Châtelier’s principle can be used to asses the effects of such changes.
– heating cobalt(II) chloride hydrate
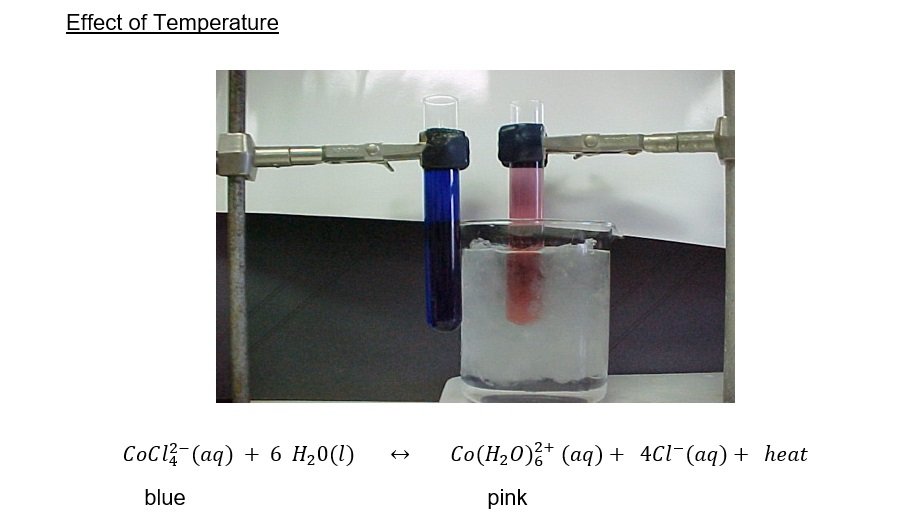
The formation of is endothermic. On heating, the equilibrium shifts to the left and the solution turns blue. On the other hand, cooling the solution favors the exothermic reaction and the solution turns pink.
– interaction between nitrogen dioxide and dinitrogen tetroxide

In general, an increase in pressure (decrease in volume) favors the reaction that decreases the total number of moles of gases (the reverse reaction) causing the solution to have a lighter color, and a decrease in pressure (increase in volume) favors the reaction that increases the total number of moles of gases (the forward reaction) causing the reaction to have a darker color.
– iron(III) thiocyanate and varying concentration of ions (ACSCH095)
Changes in Concentration
Iron (III) thiocyanate Fe(SCN)3] dissolves readily in water to give a red solution. The red solution is due to the presence of ion.

Adding some SCN- ions in the system would cause some Fe3+ ions to react with it and the equilibrium shifts from right to left and the red color of the solution deepens.
