Chemical equilibrium occurs when opposing reactions proceed at equal rates: The rate at which the products form from the reactants equals the rate at which the reactants form from the products. When additional reactant is added to a system at equilibrium, the immediate effect is to increase the concentration of reactant molecules.
According to the collision theory, this increases the number of collisions per second of reactant molecules and therefore the rate of the forward reaction.
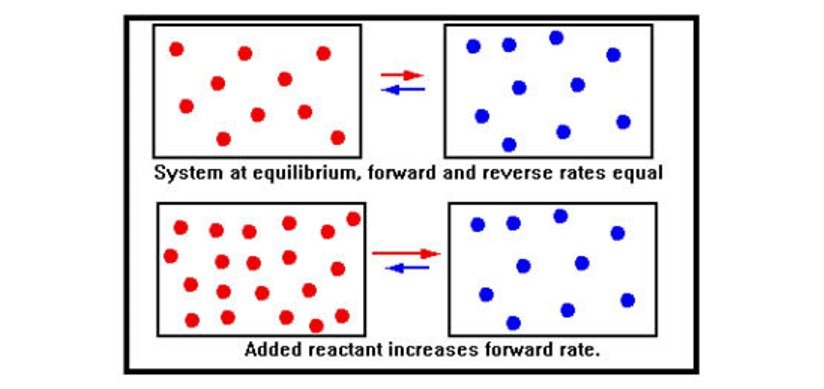
As the concentrations change, the forward rate falls, the reverse rate rises, and eventually the two rates become equal – equilibrium is reestablished with some of the added reactant having been converted to product.
