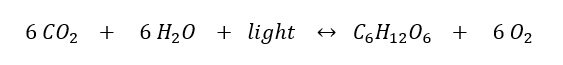Entropy (S), the measure of a system’s thermal energy per unit temperature that is unavailable for doing useful work. It is a state function just like internal energy, E and enthalpy, H. The concept of entropy provides deep insight into the direction of spontaneous change for many everyday phenomena (under Module 4).
Enthalpy (H), is defined as the internal energy plus the product of pressure, P, and volume, V, of a system (under Module 4).
– combustion reactions
For example, consider the following reaction, the combustion of methane

Here everything is gas, and we have mixtures of both elements and compounds. The standard molar entropies, So, can be used to calculate the entropy change in any reaction, at least at standard conditions of 25 °C and 1 bar (atmospheric pressure). These can be combined using the following relationship to find the entropy change for any reaction under standard conditions:
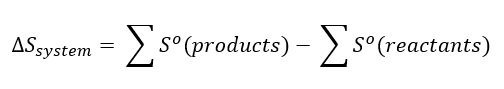
In other words, sum up all the molar entropies of all the product molecules and subtract from this the sum of all the molar entropies of all the reactant molecules which is the standard definition for change: final state – initial state.
– photosynthesis
Photosynthesis is often considered the most important chemical reaction for life on earth. Photosynthesis involves a complex series of chemical reactions, each of which convert one substance to another. These reactions taken as a whole can be summarized in a single symbolic representation as shown in the chemical equation below.