Forensic Chemistry > 2. Analysis of organic material can distinguish plant and animal material > The Carbohydrates Reducing and Non-Reducing Sugars, And Starch >
Choose equipment, plan and perform a first-hand investigation to carry out a series of distinguishing tests for the carbohydrates reducing and non-reducing sugars, and starch
Benedict’s Test
- Test for qualitative determination of reducing sugars
Iodine Test
- Test for qualitative determination of starch
Experiment
Reagents: Iodine and Benedict’s reagent (mixture of sodium carbonate, sodium citrate and copper(II) sulfate pentahydrate)
Methodology:
- Prepare two controlled test tubes of distilled water. Add Iodine solution to the 1st tube and Benedict’s solution to the 2nd Record for any observations.
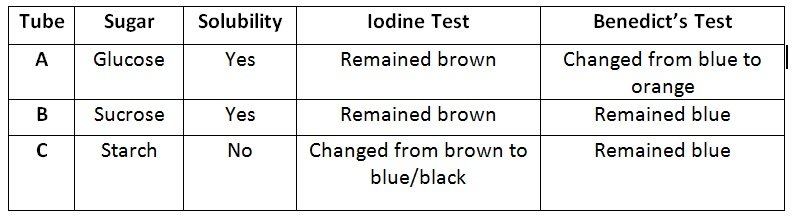
- In another 3 test tubes (Set 1), transfer 10 mL cold water to each, and add half a teaspoon of powdered glucose to tube A, sucrose to B and starch to C.
- Shake the mixture and record the solubility of each in water.
- Transfer half of the each test tube solution (5 mL) in Set 1 into separate test tubes (Set 2). To the 1st set of tubes, add iodine solution. Shake and record the colour changes in A, B and C.
- For Set 2, add Benedict’s solution to each tube. Shake and warm gently in a water bath and record for any colour changes.
Results:
Analysis of Solubility Test
- Glucose and sucrose have many polar sites capable of having hydrogen bonding with water molecules, thereby allowing them to be soluble with each other.
- Although starch has many polar sites capable of exhibiting hydrogen bonding towards water molecules, it is relatively long in which the effect of the non-polar part becomes significant. Thus, becomes insoluble with water however can absorb water molecules due to its structure and large polar parts.
Analysis of Iodine Test
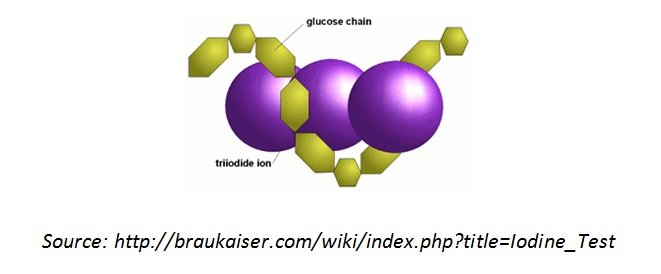
- The change in colour of starch in the presence of iodine solution is due to the formation of starch-iodide complex, specifically between the several branches in the structure of starch and iodine
Analysis of Benedict’s Test
- The glucose reduced the Benedict’s solution from Cu2+ to Cu+ and then to Cu2 Benedict’s Redox Equation:
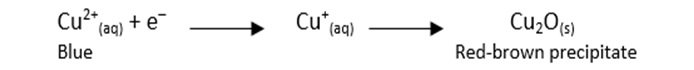
- In the structure of glucose, the aldehyde group (CHO) was oxidized by Benedict’s solution to form a carboxylic acid group (COOH), indicating that glucose is a reducing sugar

- The sucrose and starch showed no changes in color in the presence of Benedict’s reagent, indicating that these were non-reducing sugars.
