[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]Forensic Chemistry > 3. Because proteins are a major structural and metabolic component of all living organisms, the analysis of protein samples can be useful in forensic chemistry > A Distinguishing Test For Proteins > [/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Perform a first-hand investigation and gather first-hand information about a distinguishing test for proteins[/cs_text][cs_text]Biuret test
- Known test for the identification of peptides and proteins
- Involves the use of Biuret reagent which is made up of Sodium hydroxide and Copper (II) sulfate
Experiment: Protein Identification using Biuret Test
Reagent: Biuret reagent (1% CuSO4 and 10% NaOH)
Methodology:
- Place two test tubes in a test tube rack. Transfer 2 mL water in the first and 2 – mL gelatin solution in the second tube.
- Add drops of Biuret reagent in each tube.
- Compare test tube 1 (Control) with the colour of the gelatine solution in test tube 2. Record for observations
Results:
- The gelatine solution turned changes color to purple, indicating the presence of proteins or peptides
Conclusion:
- The change in colour is due to a coordination complex between the Cu2+ ions and the C—N and C=O atoms on the peptide group
[/cs_text][cs_text]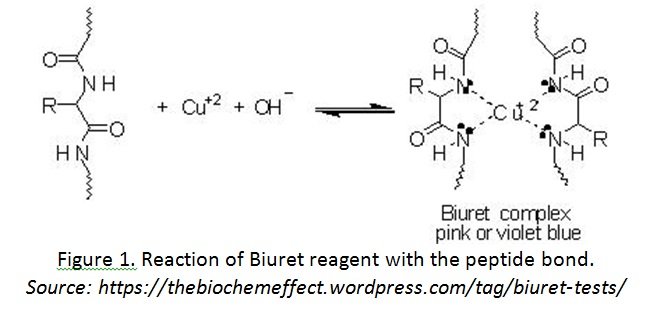 [/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]