Forensic Chemistry > 5. Much forensic evidence consists of very small samples and sensitive analytical techniques are required > How a Mass Spectrometer Operates >
Outline how a mass spectrometer operates and clarify its use for forensic chemists
- Mass spectrometry (MS) identifies the elements or compounds present in a sample by converting them to ions and then determining the mass-to-charge ratio
- The sample is vaporized and the atoms are ionized by knocking one or more electrons to form a positive ion. The ions are then accelerated in order to have a uniform kinetic energy. Then, the ions are deflected by a magnetic field according to their masses. The ions with lighter mass and ions with more positive charge experience greater deflection. The beam of ions passing through the machine is detected electrically and the read-out system displays a mass spectrum.
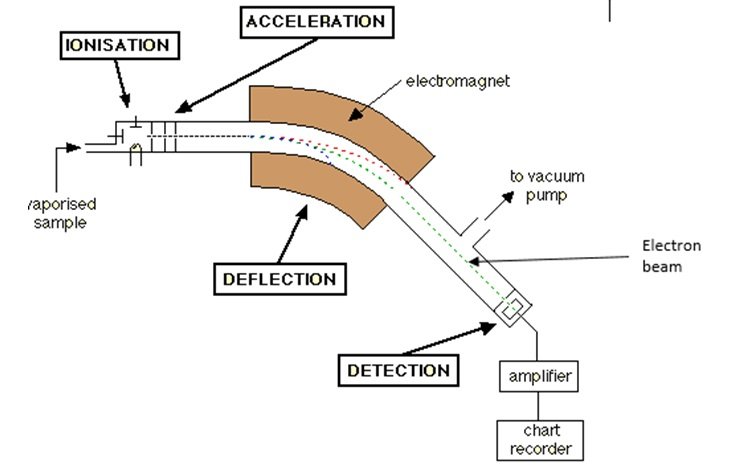
- Mass spectroscopy can be used to check whether a certain person has used a certain chemical. Mass spectrums of known drugs can be compared with that of the obtained sample.
- Mass spectroscopy can be coupled to GLC/HPLC in order to better elucidate the compounds present in a sample. Gas chromatography has a better resolution and is the preferred method of analysis for volatile and thermally stable compounds. On the other hand, HPLC can be used for thermally unstable and high molecular weight molecules.
