Industrial Chemistry > 2. Many industrial processes involve manipulation of equilibrium reactions > Interpreting The Equilibrium Constant Expression >
Interpret the equilibrium constant expression (no units required) from the chemical equation of equilibrium reactions.
Given a certain reaction at equilibrium:
aA + bB ⇌ cC + dD
where a, b, c, and d are stoichiometric coefficients for the reactive species A, B, C, and D. For the reaction at a certain temperature: 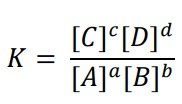
NOTES:
- It is general practice not to include units for equilibrium constants. In thermodynamics, the equilibrium constant is defined in terms of activities rather than concentrations. For an ideal system, the activity of a substance is the ratio of its concentration (or partial pressure) to a standard value, which is 1 M (or 1 atm).
- Equilibrium constant expressions are not applicable to solid and liquid species, although applicable to gases and aqueous phases. As such, the activity of pure solids and liquids is equal to 1.
