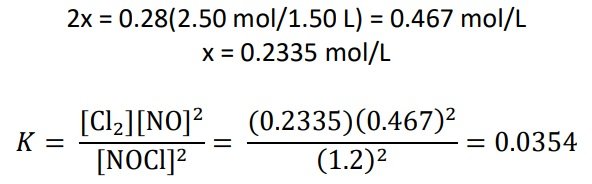Industrial Chemistry > 2. Many industrial processes involve manipulation of equilibrium reactions > Process And Present Information From Secondary Sources to Calculate K From Equilibrium Conditions >
Process and present information from secondary sources to calculate K from equilibrium conditions
A reaction vessel contains NH3, N2, and H2 at equilibrium at a certain temperature. The equilibrium concentrations are [NH3] = 0.25 M, [N2] = 0.11 M, and [H2] = 1.91 M. Calculate the equilibrium constant K for the synthesis of ammonia if the reaction is represented as
(a) N2(g) + 3H2(g) ⇌ 2NH3(g)
(b) ½ N2(g) + 3/2 H2(g) ⇌ NH3(g)
SOLUTION:
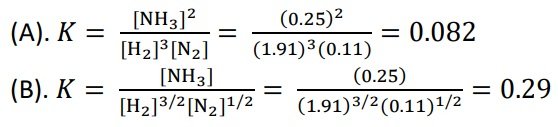
A 2.50-mole quantity of NOCl was initially in a 1.50-L reaction chamber at 400°C. After equilibrium was established, it was found that 28.0 percent of the NOCl had dissociated:
2NOCl(g) ⇌ 2NO(g) + Cl2(g)
Calculate the equilibrium constant Kc for the reaction.
SOLUTION:

For NOCl, there is a 28% decrease in the initial concentration.