[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]Industrial Chemistry > 2. Many industrial processes involve manipulation of equilibrium reactions > Temperature is The Only Factor That Changes The Value of The Equilibrium Constant (K) For a Given Equation >[/cs_text][cs_text style=”color: #800000;”]Identify that temperature is the only factor that changes the value of the equilibrium constant (K) for a given equation[/cs_text][cs_text]Changes in concentration, pressure and volume may alter the equilibrium position but it does not change the value of the equilibrium constant. However, change in temperature can alter the equilibrium constant.
Suppose that the equilibrium system given below is in a cylinder fitted with a movable piston.
CH4(g) + H2O(g) ⇌ CO2(g) + 3H2(g) (endothermic)
- The forward reaction is ENDOTHERMIC (the system ABSORBS HEAT):heat + CH4(g) + H2O(g) ⇌ CO2(g) + 3H2(g)
- The reverse reaction is EXOTHERMIC (the system RELEASES HEAT):
CO2(g) + 3H2(g) ⇌ CH4(g) + H2O(g) + heat
- At equilibrium at a certain temperature, the heat effect is ZERO because there is no net reaction.
- If we treat heat as if it is a chemical reagent, then a rise in temperature ‘adds’ heat to the system whereas a decrease in temperature ‘removes’ heat from the system.
- As with a change in any other parameter (concentration, pressure, or volume), the system shifts to reduce the effect of the change.
- Therefore, a temperature increase favors the endothermic direction of the reaction (from left to right of the equilibrium equation), which decreases [CH4] and [H2O] and increases [CO2] and [H2].
- A temperature decrease favors the exothermic direction of the reaction (from right to left of the equilibrium equation), which decreases [CO2] and [H2] and increases [CH4] and [H2O].
- Consequently, the equilibrium constant, given by
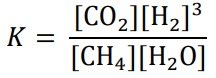 increases when the system is heated and decreases when the system is cooled.
increases when the system is heated and decreases when the system is cooled.
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]