[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]Industrial Chemistry > 5. Saponification is an important organic industrial process > The Soap as an Emulsifier > [/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Explain that soap, water and oil together form an emulsion with the soap acting as an emulsifier[/cs_text][cs_text]Emulsion – is the dispersion of small droplets of one liquid within another liquid.
Ex. soap + water + oil
Emulsifier – a substance which allows the initially immiscible substances to be miscible with each other
Ex. soap
Soap as an emulsifier:
- When oil and water are mixed together, no interaction will occur and two distinct layers will be observed since oil is non polar and the water is polar.
- However, a cloudy layer is observed between the oil and water layers when soap is added as a result of emulsion.
- The hydrophobic tail of the soap molecules interact with the oil molecules, causing the oil molecule to break into smaller droplets (micelles) which are suspended in water and seen in cloudy layer.
Note: Oil micelles do not clump together since the anionic heads of adjacent soap molecules forms repulsion towards each other (electrostatic attraction).[/cs_text][cs_text]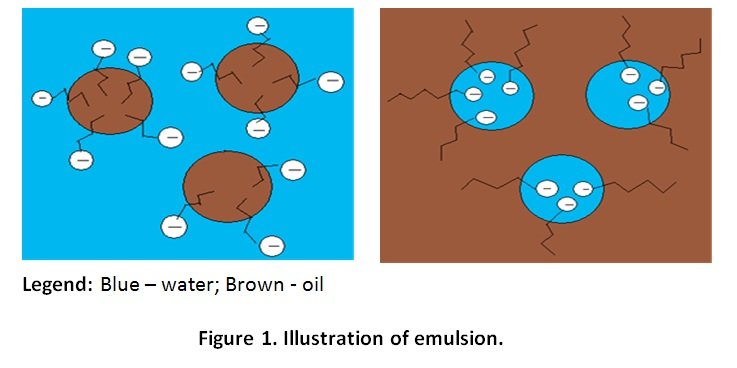 [/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]