Industrial Chemistry > 4. The industrial production of sodium hydroxide requires the use of electrolysis > The Steps in The Industrial Production of Sodium Hydroxide >
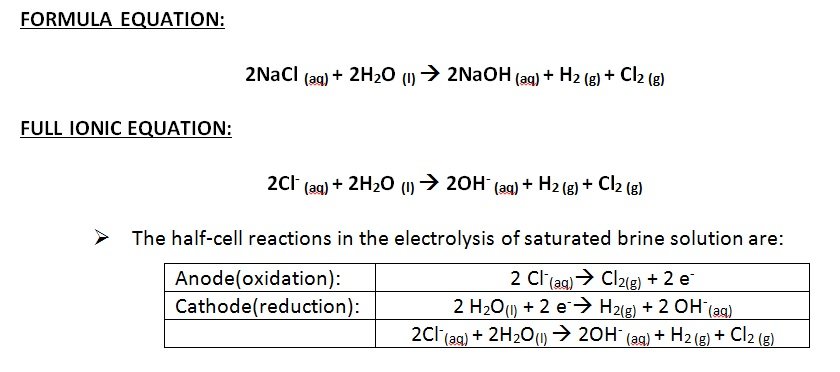
Outline the steps in the industrial production of sodium hydroxide from sodium chloride solution and describe the reaction in terms of net ionic and full formulae equations
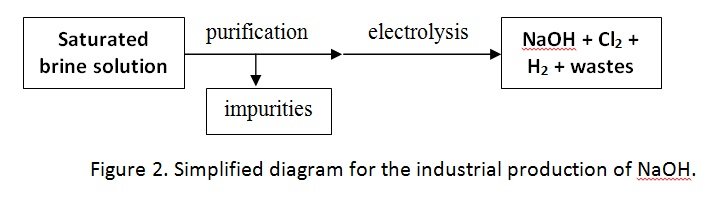
- Sodium hydroxide is produced industrially through the electrolysis of brine (sodium chloride) solution.
STEPS IN INDUSTRIAL PRODUCTION OF NaOH
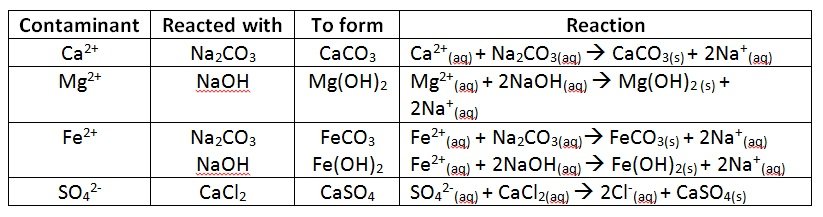
- PURIFICATION
- Water used to dissolve the salt must be purified and softened
- Impurities in the saturated brine solution is removed by precipitation process.
- The precipitate formed is removed as a sludge.
- ELECTROLYSIS
- The purified brine solution undergoes electrolysis in order to produce NaOH and other gases such as Cl2 and H2
- The chlorine ions in the salt water solution are oxidized to form chlorine gas and the water in the salt water solution is reduced to form hydroxide ions and hydrogen gas
- Electrons are lost from chlorine ions and these electrons are gained by the water molecules