Industrial Chemistry > 4. The industrial production of sodium hydroxide requires the use of electrolysis > The Three Electrolysis Methods >
Distinguish between the three electrolysis methods used to extract sodium hydroxide: mercury process, diaphragm process, membrane process by describing each process and analysing the technical and environmental difficulties involved in each process
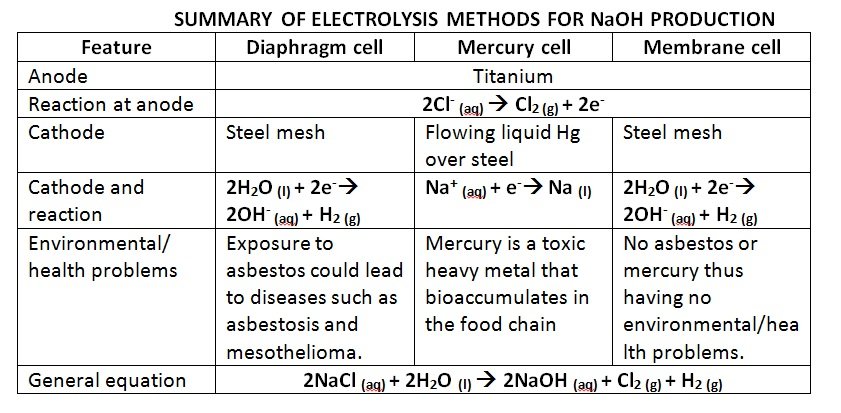
DIAPHRAGM PROCESS
- first process used to manufacture sodium hydroxide
- made up of porous mixture of asbestos and polymers
- solution can seep through from the anode compartment to the cathode side
- higher level of liquid on the anode side makes sure that the flow of liquid is from anode to cathode – prevents any NaOH solution formed to return to anode
- NaOH solution leaving the cell is concentrated by evaporation
- evaporation process leads to crystallization of NaCl impurities which can be dissolved and be passed through the cell again
Key Features:
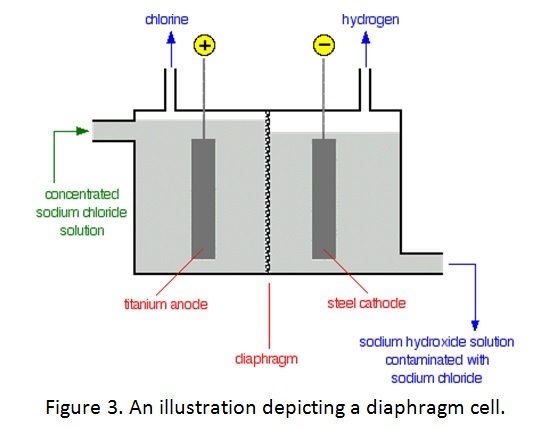
- Anode: titanium (to resist chlorine attack)
- Cathode: steel mesh (to not react with OH–)
- Anode and cathode compartment separated by a porous asbestos diaphragm(to prevent the products and reactants from mixing)
- Anode reaction: 2Cl– (aq) à Cl2 (g) + 2e–
- Cathode reaction: 2H2O (l) + 2e–à 2OH– (aq) + H2 (g)
- Electrolyte: brine (salt water)
Process:
- the concentrated sodium chloride solution is entered into the cell
- at the titanium anode, chlorine ions from solution lose electrons and are oxidised to form chlorine gas, which ca be collected and removed
- at the steel mesh cathode, water molecules are reduced to form hydroxide ions and hydrogen gas, which can also be removed
- the sodium ions in NaCl solution react with the hydroxide ions to produce sodium hydroxide (some contamination by NaCl) which is then collected by evaporation
Technical/Environmental Difficulties:
- exposure to airborne asbestos fibres could lead to mesothelioma and asbestosis
- leakage inside the diaphragm would cause the hydrogen to react explosively with chlorine or oxygen
- contact between hydroxide ion and chlorine ion in solution would lead to unwanted hypochlorite formation
- reducing the amount of chloride inpurities present in the final hydroxide solution
- largeamounts of electric currents could create heat and magnetic effects.
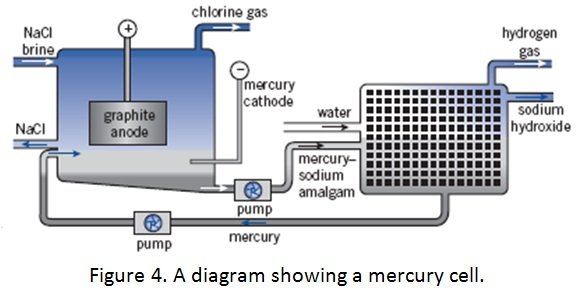
MERCURY CELL
- uses liquid mercury as a cathode and usually titanium as the anode
- sodium from the brine solution forms an amalgam with Hg and is removed from the cell
- the amalgam reacts with water which leads to formation of NaOH
Key Features:
- Anode: titanium (to resist attack)
- Cathode: flowing mercury over steel (used to prevent water being reduced, instead allowing sodium ions to be reduced).
- Anode reaction: 2Cl– (aq) à Cl2 (g) + 2e–
- Cathode reaction: Na+ (aq) + e–à Na (l)
- Decomposer – decomposes mercury/sodium amalgam with water to produce sodium hydroxide
- Electrolyte: brine (salt water)
Process:
- brine is flowed slowly through the tank
- sodium ions are reduced at the cathode and the sodium metal produced dissolves in the mercury to produce an amalgam
- amalgam flows through the cell until arrival at the decomposer where it reacts with water to produce sodium hydroxide:
2Na/Hg (l) + 2H2O (l) 2NaOH (aq) + H2 (g) + 2Hg(l)
- hydrogen is collected and stored and is a valuable by-product of the reaction
- Hgis recycled and is reused as the cathode because it does not react with water
- chlorine gas, which is produced at the anode, is also removed
Technical/Environmental Difficulties:
- Improper disposal of Hg in bodies of watercan lead to consumption by aquatic organisms. This may lead to devastating effects on the organisms because mercury biomagnifies and bioaccumulates along the food chain. The mercury could badly injure or kill the aquatic organisms.
- large amounts of electric currents create heat and magnetic effects.
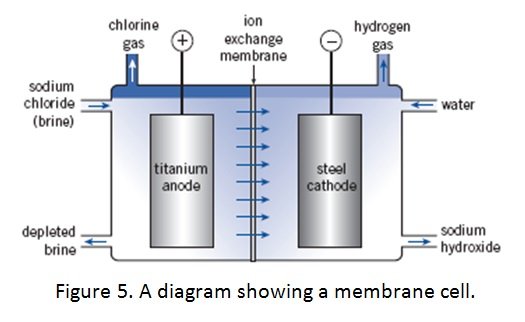
MEMBRANE CELL
- widely used method to produce sodium hydroxide
- the membrane is made from a polymer which allows positive ions to pass through it
- the ion-selective, impermeable membrane prevents the interaction of chloride ions with hydroxide ions which can lead to hypochlorite formation
- the sodium chloride solution has to be pure to prevent other positively charged impurities from passing through the membrane and contaminating the NaOH solution
Key Features:
- Anode: titanium (to resist attack)
- Cathode: steel mesh
- an ion-selective, water impermeable membrane made out of a synthetic polymer such as Teflon
Process:
- brine is entered into anode compartment of the cell and water is entered into cathode compartment of cell
- at the anode, chlorine ions from the brine are oxidized to form chlorine gas which is collected and removed
- at the cathode, water molecules are reduced to form hydroxide ions and hydrogen gas, which is collected and removed
- membrane allows sodium ions to pass through to cathode compartment to react with the hydroxide ions to produce sodium hydroxide, which is then removed and further purified
Technical/Environmental Difficulties:
- the process has negligible technical and environmental difficulties