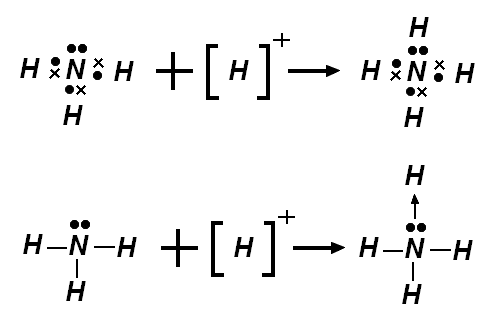Monitoring and Management > 4. The Atmosphere >
Describe the formation of a coordinate covalent bond
- Covalent bond: A chemical bond formed between two atoms through the sharing of pairs of electrons.
- Single covalent bond: A covalent bond involving the sharing of one pair of electrons.
- Double covalent bond: A covalent bond involving the sharing of two pairs of electrons.
- An oxygen molecule contains a double covalent bond.
- Coordinate covalent bond: A covalent bond in which one of the two atoms bonded supplies all of the shared electrons.
- Coordinate covalent bonds are formed when one atom does not have a complete outer shell, while another atom does have a complete outer shell and has at least one unshared electron pair.
- Once formed, coordinate covalent bonds are indistinguishable from normal covalent bonds.
Example of coordinate covalent bonding