Monitoring and Management > 4. The Atmosphere >
Identify and name examples of isomers (excluding geometrical and optical) of haloalkanes up to eight carbon atoms
Gather, process and present information from secondary sources including simulations, molecular model kits or pictorial representations to model isomers of haloalkanes
- Haloalkane: An alkane containing at least one halogen functional group.
- CFCs and halons are examples of haloalkanes.
- Isomer: One of a series of compounds that have the same molecular formula, but different structural formulae.
- Haloalkanes are named using the following system:
- Haloalkanes are named similarly to their corresponding alkanes, ending with “ane” preceded by a prefix denoting the number of carbon atoms in the longest carbon atom chain:
| Number | One | Two | Three | Four | Five | Six | Seven | Eight |
| Prefix | Meth | Eth | Prop | But | Pent | Hex | Hept | Oct |
- Halogen functional groups are identified by prefixes:
| Halogen | Bromine | Chlorine | Fluorine | Iodine |
| Prefix | Bromo | Chloro | Fluoro | Iodo |
- If there is more than one possible position for a functional group, then its position must be identified by a number.
- The chain is numbered from the end that leads to the smallest sum when all of the substituent numbers are added.
- If there is more than one of a particular kind of functional group, then the functional group prefix must be preceded by another prefix:
| Number | Two | Three | Four |
| Prefix | Di | Tri | Tetra |
- If there is more than one type of functional group, then they are arranged in alphabetical order according to the name of the halogen.
- If the above rules give more than one possible name, then the correct name is the one that gives the lowest numbers to the most electronegative halogen.
| Name | Structural Formula |
| Trichloromethane | 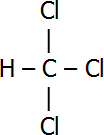 |
| 1,1-dibromo-2-chloroethane | 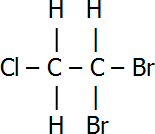 |
| 1,2,2-trichloro-1,1,2-trifluoroethane | 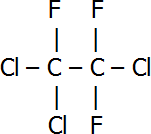 |
Examples of haloalkane names
| Name | Structural Formula |
| 1,2,2-trichloro-1,1,2-trifluoroethane | 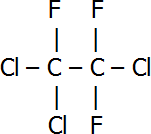 |
| 1,1,1-trichloro-2,2,2-trifluoroethane | 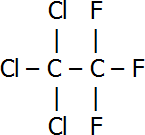 |
An example of a set of isomers
- The above isomers can be made with a molecular modelling kit.
