Monitoring and Management > 4. The Atmosphere >
Identify the origins of chlorofluorocarbons (CFCs) and halons in the atmosphere
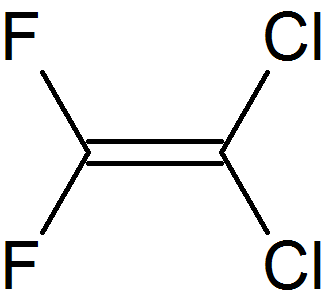
- Chlorofluorocarbon (CFC): A haloalkane that contains both fluorine and chlorine atoms, and no hydrogen atoms.
- Halon: Also referred to as a brominated CFC, a haloalkane that contains bromine, chlorine and/or fluorine atoms, and no hydrogen atoms.
- CFCs were developed in the 1930s to replace ammonia as refrigerants.
- CFCs became extensively used as:
- Refrigerants.
- Solvents in dry cleaning.
- Propellants in spray cans.
- Blowing agents for expanded plastic products.
- Halons were developed for the extinguishing of electrical fires.
- Halons became widely used for this purpose, but were never used as extensively as CFCs.
- Through their various uses, CFCs and halons were released into the atmosphere.
- CFCs diffuse into the stratosphere because:
- They are not destroyed by sunlight and oxygen in the troposphere.
- They are insoluble in water, and are thus not ‘washed out’ of the atmosphere.