Monitoring and Management > 4. The Atmosphere >
Compare the properties of the gaseous forms of oxygen and the oxygen free radical
- Free radical: A species containing at least one unpaired electron.
- An oxygen free radical has six electrons in its outer shell, with two of these being unpaired:
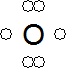
- The unpaired electrons make radicals very reactive.
- When ultraviolet radiation splits an oxygen molecule, two oxygen free radicals are formed.
- Oxygen free radicals are more reactive than ozone molecules, which are more reactive than oxygen molecules.
