Shapes of alkane, alkene and alkyne molecules
| Type of compound | Hybridization | Structure/Shape of molecules | Bond Angles |
|---|---|---|---|
| Alkane | sp3 | Tetrahedral | 109.5° |
| Alkene | sp2 | Trigonal Polar | 120° |
| Alkyne | sp | Linear | 180° |
Reference image:
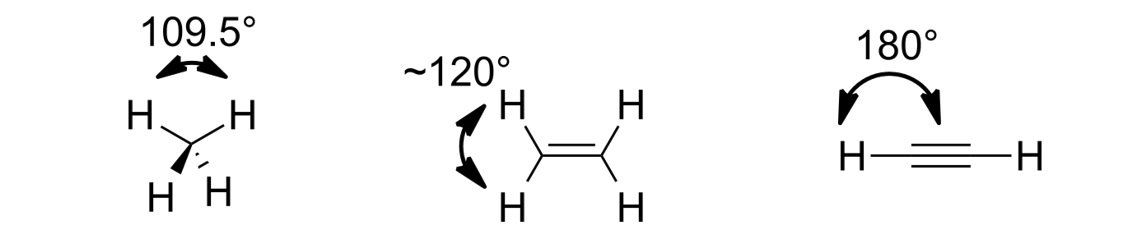
Shapes of alkane, alkene and alkyne molecules
| Type of compound | Hybridization | Structure/Shape of molecules | Bond Angles |
|---|---|---|---|
| Alkane | sp3 | Tetrahedral | 109.5° |
| Alkene | sp2 | Trigonal Polar | 120° |
| Alkyne | sp | Linear | 180° |
Reference image:
