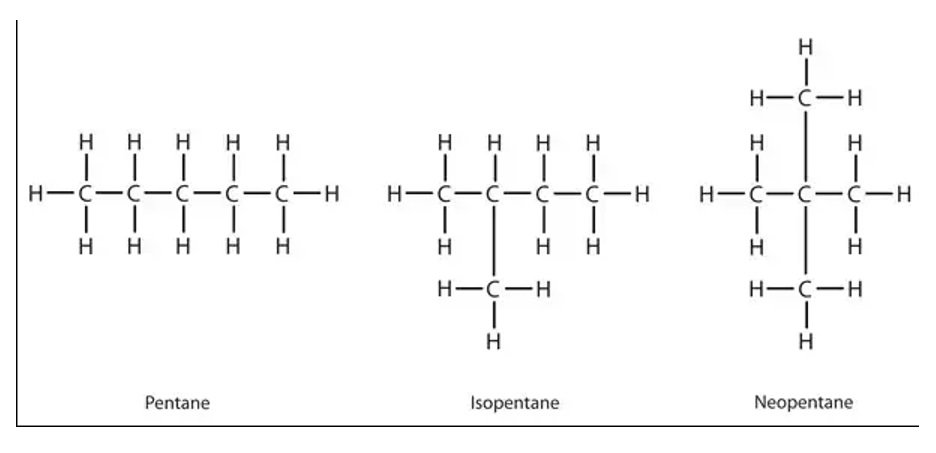
Chain Isomers
- Rearrangement in the backbone carbon molecules in hydrocarbons and the resultant products being compounds having same molecular formula and but different structural formula.
- For example, 3 different isomers can be made from pentane which are n-pentane (generally termed as pentane), isopentane (2-methylbutane) and neopentane (dimethylpropane).
Reference image for structures
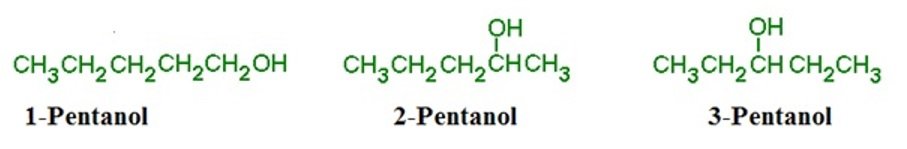
Position Isomers
- Reordering and changes in position of functional groups in molecules.
- For example, pentanol can have 3 position isomers, 1-pentanol, 2-pentanol and 3-pentanol respectively.
Reference image for structures
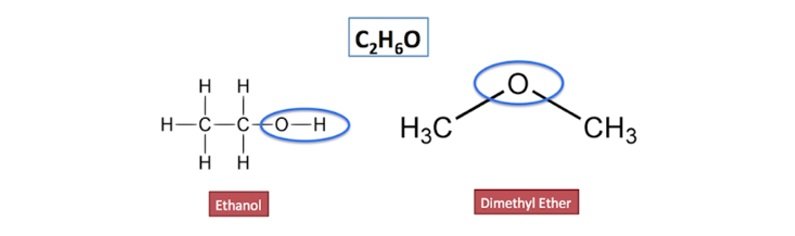
Functional Group Isomers
- Compounds having same molecular formula but different functional groups in their structure are known as functional isomers.
- Difference in functional groups refers to the fact that functional isomers belong to different homologous series.
Reference image for structures