Nylon
Structure
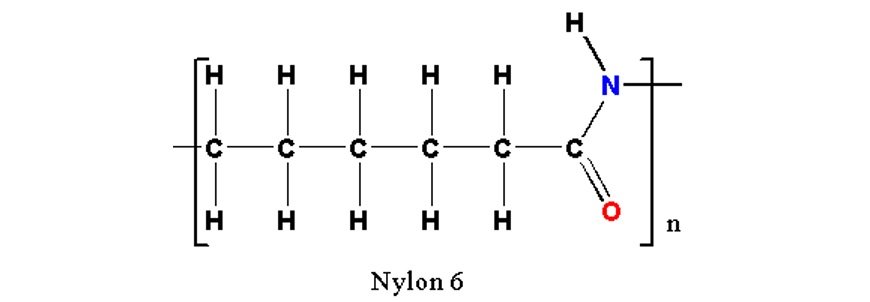
- Condensation polymer derived from condensation reaction of monomers containing terminal carboxylic acid and amine groups.
- The monomers form repeating units and alternate in the chain.
Reference image for structure
Properties
- Strong, elastic and easy to wash.
- Have the ability to retain shape even after heavy strain.
- Possesses excellent abrasion resistance and colour lightfastness.
- High resistance to insects, fungi, animals, as well as molds, mildew, rot and many chemicals.
Uses
- In manufacture of parachutes.
- Common material for production of different forms of clothing, fabrics and ropes.
- Nylon resins are used in automobile and food industries.
Polyesters
Structure
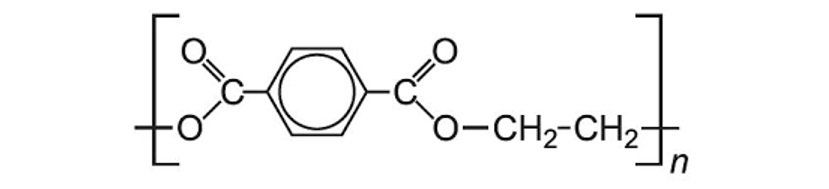
- Polymers formed from a dicarboxylic acid and a diol, also known as Polyethylene Terephthalate.
- Being an ester, it is made from an acid, benzene-1,4-dicarboxylic acid (terephthalic acid), and an alcohol, ethane-1,2-diol.
- Each repeating unit of monomer is held with an ester linkage.
Reference Image for structure:
Properties
- Formation of highly effective Van der walls force and hydrogen bonds provide good tenacity.
- The hydrophobic nature of the polymer system attracts fats, oils, grease, acids etc.
- Poor heat conductor and resistant to UV radiation from the sun.
Uses
- Fabrics woven from polyester are used in apparel and home furnishing.
- Industrial polyester fibers, yarns and ropes are used in car tire reinforcements, fabrics for conveyor belts, safety belts, coated fabrics and plastic reinforcements with high-energy absorption.
- Polyesters are also used to make bottles, films, tarpaulin, canoes, liquid crystal displays, holograms, filters, dielectric film for capacitors, film insulation for wire and insulating tapes.
Reference:
