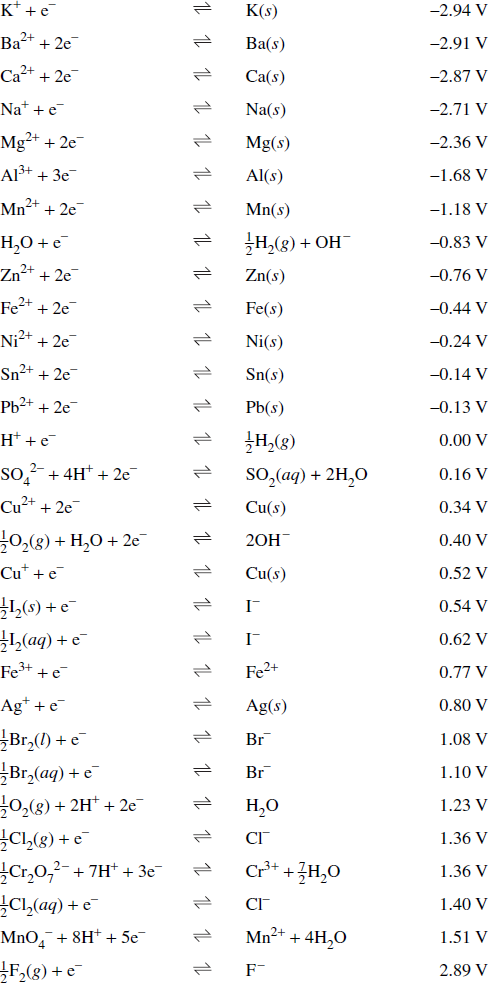Production of Materials > 4. Electrochemical Methods >
Solve problems and analyse information to calculate the potential (E°) requirement of named electrochemical processes using tables of standard potentials and half-equations
- Table of standard potentials: A table that lists common reduction half-equations along with the potentials of the half-equations, known as reduction potentials, relative to the standard hydrogen electrode.
- Oxidation potentials (potentials of the reverse half-equations to those listed) are equal in magnitude but opposite in sign to reduction potentials.
- The overall potential of an electrochemical process can be calculated by adding the reduction potential to the oxidation potential:
- If the potential is positive, then the reaction is spontaneous (i.e. it is a galvanic cell).
- If the potential is negative, then the reaction requires the application of a voltage greater than or equal to the magnitude of the potential (i.e. it is an electrolytic cell).
Table of Standard Potentials:
Extract from HSC Chemistry Data Sheet. © 2010, Board of Studies NSW.