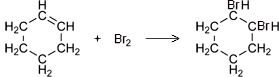[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]Production of Materials > 1. Fossil Fuel Products >
Identify data, plan and perform a first-hand investigation to compare the reactivities of appropriate alkenes with the corresponding alkanes in bromine water[/cs_text][/cs_column][/cs_row][/cs_section][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]
- Bromine water has a distinctive brown colour.

- Saturated: Containing only single carbon-to-carbon bonds.
- Unsaturated: Containing at least one double carbon-to-carbon bond.
- Alkenes react spontaneously with bromine water due to their unsaturated nature.
- The reaction between an alkene and bromine involves the breaking open of the alkene’s double bond and the inclusion of bromine in its structure (an addition reaction).
- This results in the production of an alkane with two bromine functional groups.
- Therefore, when alkenes come into contact with bromine water, they cause it to decolourise.
- Alkanes do not react with spontaneously bromine water due to their saturated nature.
- Bromine is non-polar and therefore dissolves more readily in a non-polar alkane than in polar water.
- Therefore, when alkanes come into contact with bromine water, they cause it to decolourise, while they adopt the colour.
- If placed in ultraviolet light, a substitution reaction may occur between an alkane and bromine water.
- This results in the production of hydrogen bromide and an alkane with a single bromine functional group.
- Therefore, when alkanes come into contact with bromine water and are exposed to ultraviolet light, both substances decolourise.
- Cyclohexane and cyclohexene can be used to demonstrate these reactions.
[/cs_text][/cs_column][/cs_row][/cs_section][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][x_video_embed no_container=”false” type=”16:9″][/x_video_embed][/cs_column][/cs_row][/cs_section][/cs_content]