Production of Materials > 1. Fossil Fuel Products >
Identify that ethylene, because of the high reactivity of its double bond, is readily transformed into many useful products
- The double bond present in alkenes, such as ethylene, makes them much more reactive than their corresponding alkanes.
- Many substances react with alkenes through breaking open the double bond into two single bonds, this being an example of an addition reaction.
- Addition Reaction: A reaction in which two or more molecules are reacted together to form a single molecule.
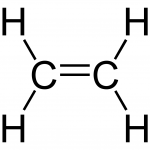

Structure of ethylene, with its double bond between carbon atoms
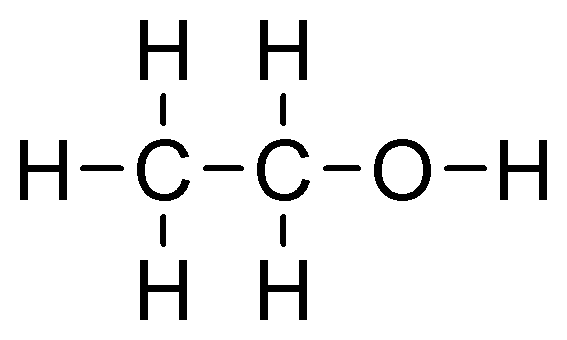
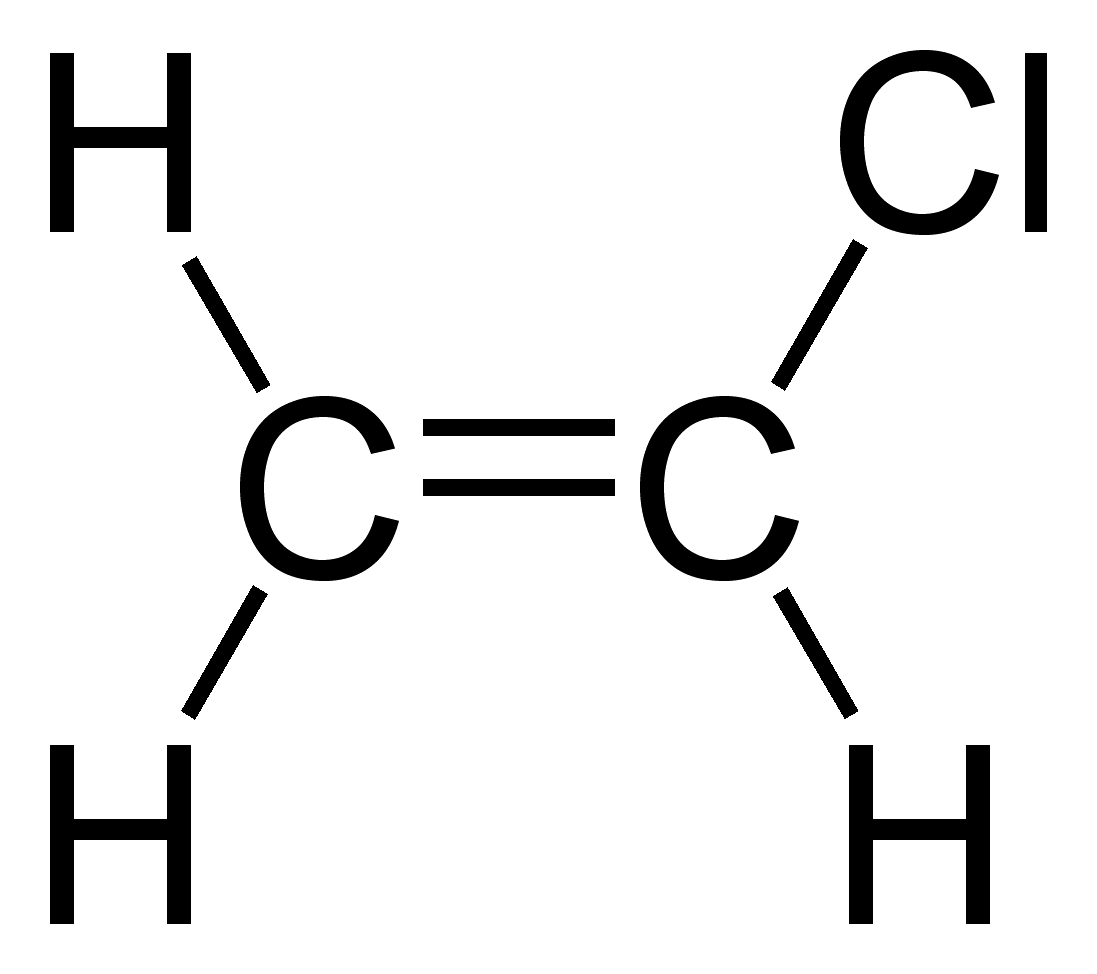
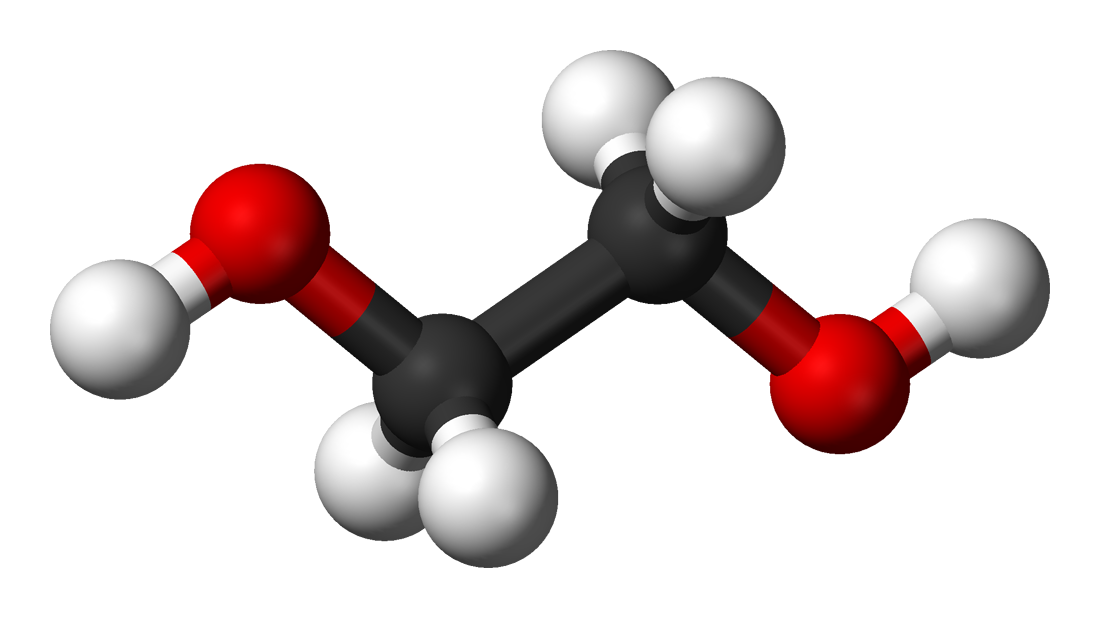
| Product | Ethanol | Ethylene oxide | Ethylene glycol | Vinyl chloride |
| Reactants | Ethylene and water | Ethylene and oxygen | Ethylene oxide and dilute acid | Oxygen and chlorine |
| Conditions | – High pressure – About 300˚C – Phosphoric acid catalyst | About 250˚C with silver as a catalyst | Treatment with dilute acid solution | – About 150˚C – Copper chloride catalyst |
| Uses | – Fuel – Industrial, commercial and domestic solvent – Cleaning and disinfecting fluid | Fumigant | – Anti-freeze liquid – Manufacturing of polyester | Monomer for producing polyvinyl chloride |
| 2D structure diagram | ||||
| 3D structure diagram |
Products of ethylene