[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]Shipwrecks and Salvage > 1. Oceans and Electrolytes >
Identify that oxidation-reduction reactions can occur when ions are free to move in liquid electrolytes[/cs_text][/cs_column][/cs_row][/cs_section][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]
- As well as the movement of electrons, the movement of ions is an important part of oxidation-reduction reactions.
- Electrolyte: A substance which, when molten or in solution, separates into ions and conducts electricity.
- The strength of an electrolyte is determined by its solubility in water, as the more soluble a substance is, the more ions are available to transmit current.
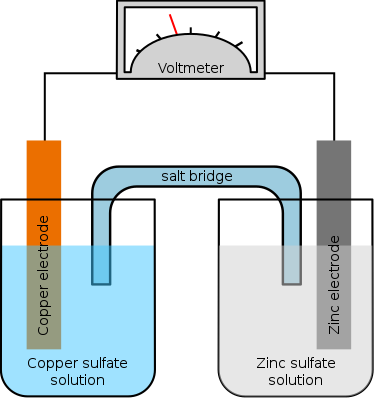
- The importance of an electrolyte in ion transfer is seen in the following two scenarios:
- If a metal is coated with an ionic compound of a less reactive metal, the oxidation-reduction reaction will only occur on the surface where the metal makes contact with the compound.
- If a metal is placed in an aqueous solution containing the ions of a less reactive metal, the oxidation-reduction reaction would be quite rapid because:
- The less reactive metal ions are free to move towards the surface of the more reactive metal and accept electrons.
- Once formed, the more reactive metal ions are able to diffuse away and expose a new surface for reaction.
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]