The Acidic Environment > 4. Acid/Base Definitions >
Perform a first-hand investigation and solve problems using titrations and including the preparation of standard solutions, and use available evidence to quantitatively and qualitatively describe the reaction between selected acids and base
What follows is an extensive experimental report about an analysis of different types of lemon juice. For a simpler overview of titration technique, click here.
Introduction
Being a citrus fruit, lemon and its juice contain citric acid as by far the dominant acid. Indeed, it is this acid that provides much of the flavour that we recognise as being distinctly lemon, and the citric acid content of a particular lemon juice can have a great impact on how that juice tastes.
The citric acid content of a lemon juice can be determined through a titration of the juice. This process involves a certain volume of juice having a base of a known concentration added to it until a point is reached where all of the acid in the juice is neutralised (known as the endpoint). Citric acid is a weak acid, and is thus best titrated using a strong base, such as sodium hydroxide, with the combination of a weak acid and a strong base producing an endpoint that corresponds to the range of phenolphthalein indicator (pH range of 8.3-10).
From the results of the titration, the amount of citric acid in the juice can be determined.
The citric acid content of lemon juice varies between lemons, with a typical concentration being about 5% by mass . Most bottled lemon juice companies standardise the citric acid content of their juice to a certain figure during the reconstitution process.
Berri Squeeze was the brand of bottled juice used in my experiment. I contacted Berri Juices after performing the experiment, and found that they standardise the citric acid content of their juice to 5 grams per 100 mL (although Berri stated that this could vary from a minimum of 4 grams to a maximum of 5.2 grams).
Aim
To volumetrically analyse both freshly squeezed lemon juice and bottled lemon juice (through titration) and compare their citric acid contents.
Apparatus
- Lemons.
- Lemon squeezer.
- Bottled lemon juice (Berri Squeeze).
- Straining gauze.
- Sodium hydroxide of approximately 1 mol./L.
- Sodium hydrogen sulfate powder.
- Phenolphthalein indicator.
- Distilled Water.
- Electronic scales.
- Retort stand and clamp.
- Two 250 mL beakers.
- 50 mL burette.
- 200 mL volumetric flask.
- 250 mL conical flask.
- 25 mL pipette.
- Glass funnel.
Method
Standardisation of sodium hydroxide
- Determine the mass of sodium hydrogen sulfate that would be required to form 200 mL of a 1 M solution.
- Weight this mass and transfer it to the 200 mL volumetric flask.
- Add about 100 mL of distilled water to the flask and swirl until all of the sodium hydrogen sulfate has dissolved.
- Fill the flask to the calibration point with more distilled water.
- Measure exactly 25 mL of the sodium hydrogen sulfate solution into the 250 mL conical flask using the pipette.
- Add a few drops of phenolphthalein indicator to the sodium hydrogen sulfate solution (the solution should remain clear).
- Fill the burette to the top calibration mark with the sodium hydroxide solution.
- Add the sodium hydroxide solution to the sodium hydrogen sulfate solution until the phenolphthalein indicator turns red, indicating that the endpoint has been reached, and record the amount of sodium hydroxide used.
- Repeat steps 5 to 8 three times, refilling the burette and cleaning the conical flask each time.
Titration of freshly squeezed lemon juice with sodium hydroxide (using phenolphthalein indicator)
- Squeeze the lemons and strain the lemon juice to remove any pulp.
- Measure exactly 25 mL of lemon juice into the 250 mL conical flask using the pipette.
- Add about 75 mL of distilled water to the lemon juice.
- Add a few drops of phenolphthalein indicator to the lemon juice and distilled water (the solution should remain clear).
- Fill the burette to the top calibration mark with the sodium hydroxide solution.
- Add the sodium hydroxide solution to the lemon juice until the phenolphthalein indicator turns red, indicating that the endpoint has been reached, and record the amount of sodium hydroxide used.
- Repeat steps 2 to 6 three times, refilling the burette and cleaning the conical flask each time.
Titration of bottled lemon juice with sodium hydroxide (using phenolphthalein indicator)
- Strain the bottled lemon juice if necessary.
- Repeat steps 2 to 7 in the procedure above, substituting freshly squeezed lemon juice with bottled lemon juice.
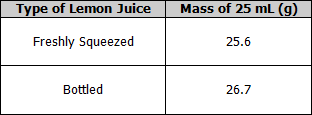
Measurement of the mass of 25 mL of lemon juice
- Place a beaker on the electronic scales and zero the scales.
- Pipette 25 mL of freshly squeezed lemon juice into the beaker.
- Record the mass.
- Repeat for bottled lemon juice.
Raw Results
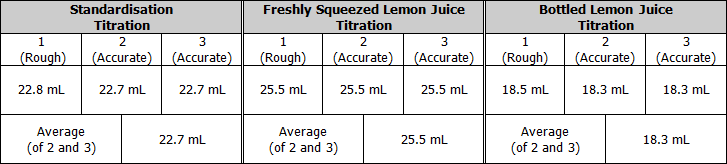
Relevant Chemical Equations
1. Standardisation of sodium hydroxide
![]()
1. Titration of lemon juice
![]()
Calculations
Primary standard
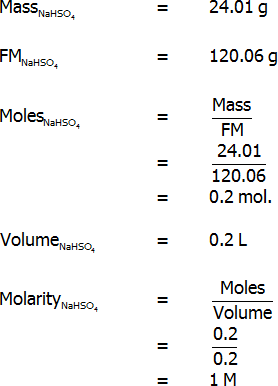
Standardisation titration

Titration of lemon juice – freshly-squeezed (left) and bottled (right)

Percentage of citric acid (by mass) – freshly-squeezed (left) and bottled (right)
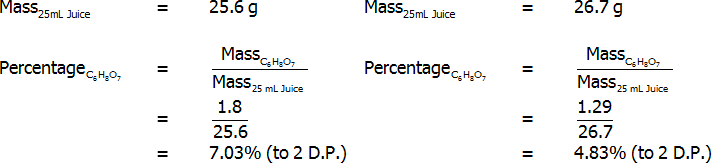
Percentage of citric acid (by volume) – freshly-squeezed (left) and bottled (right)
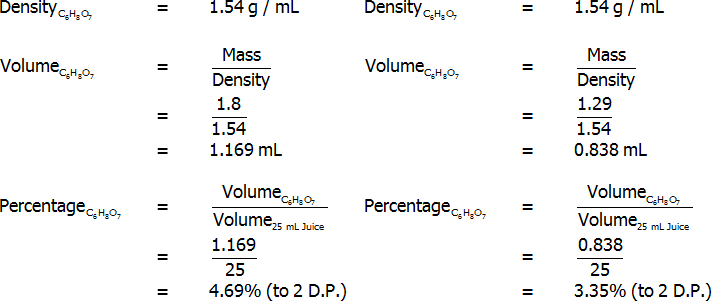
Percentage of citric acid (grams per 100 mL) – freshly-squeezed (left) and bottled (right)
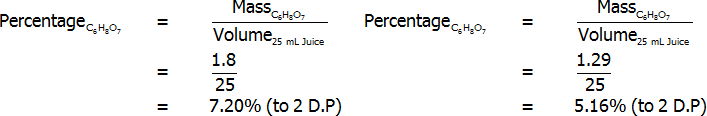
Results
Calculation of Error
The only result for which any sort of error can be determined is the bottled lemon juice citric acid percentage, or more specifically, the percentage in grams per 100 mL (as this is the type of percentage that Berri gave). Berri stated that the concentration of their bottled juice is typically 5%. Assuming that this is true:
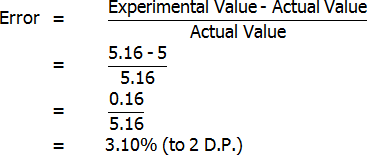
However, Berri also stated that citric acid percentages could range from as low as 4% to as much as 5.2% (grams per 100 mL). My value of 5.16% is just within this range, and therefore it is possible that my results may not even have had any error (although this is unlikely). Because my result is almost at the top of the range, it is most likely that my experimental value is greater than the actual value. I will make this assumption in discussing the reliability of my results.
Conclusion
Both freshly squeezed and bottled lemon juices were successfully analysed through titration. The bottled lemon juice was found to contain 4.83% citric acid by mass, 3.35% citric acid by volume, and 7.20% citric acid in grams per 100 mL. In contrast, the freshly squeezed lemon juice was found to contain a surprisingly high 7.03% citric acid by mass, 4.69% citric acid by volume, and 5.16% citric acid in grams per 100 mL. This value of 5.16 grams per 100 mL for the bottled juice represents an error of 3.10%, assuming that the actual value is 5 grams per 100 mL. Overall, reasonably accurate results were found, and the freshly squeezed variety of juice was found to have a much higher citric acid content that the bottled variety.
Problems Encountered / Reliability of Results
- There would have been very small amounts of acids other than citric acid in the lemon juice, meaning that the percentage of citric acid is actually slightly lower. For example, ascorbic acid would have been present in small quantities.
- Some of these other acids may have been strong acids, which would have affected the endpoint (the accuracy of the endpoint relied upon the assumption that all of the acids in the lemon juice were weak, like citric acid).
- Because lemon juice is coloured, it is more difficult to observe the change in colour of phenolphthalein indicator at the endpoint.
- In between titrations, extremely small amounts of water would have evaporated from the sodium hydroxide solution, as well as the lemons, which may have affected results very slightly.
- Despite straining the lemon juice before titration, small amounts of pulp would have affected the titration.
Possible Improvements
- One possible improvement could have been to add an extra part to the experiment, involving the formation of a calcium citrate precipitate from the citric acid in the lemon juice (using limewater of a known molarity), which could be extracted and weighed:
- This would allow the determination of the percentage of citric acid in the lemon juice specifically, rather than the total acidity. The results of this could have been compared to those of the titration, and the contribution of citric acid to the overall acidity could have been determined.
- The experiment could have been performed over one single day (rather than over five days), which would have greatly reduced any loss of water from the sodium hydroxide solution and the lemon juice, which may have improved results slightly.
- Instead of straining the lemon juice, it could have been filtered to remove all pulp and thus increase the accuracy of the result.

