[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]The Chemistry of Art > 5. Complexes > Chelated Ligands >[/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Identify examples of chelated ligands[/cs_text][cs_text]
- Ligands are classified according to the manner in which they bind to the central metal ion
- Ligands that bond using the electron pair of a single donor atom
- Monodentate Ligands (One toothed)
- g H2O, Cl–, CN–
[/cs_text][cs_text]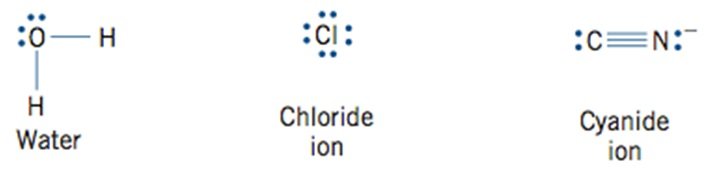 [/cs_text][cs_text]
[/cs_text][cs_text]
- Ligands that bond through electron pairs on more than one donor atom
- Polydentate Ligands
- Polydentate ligands are also known as chelating (Claw) agents
- Multi-point attachment resembles a crab claw
- Donor atoms bond simultaneously to the central metal ion
- g oxalate ion, Ethylenediamine
[/cs_text][cs_text]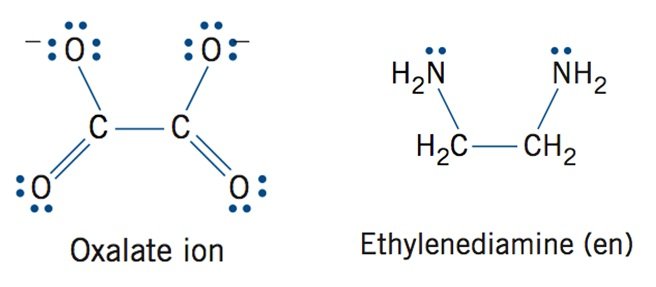 [/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]