The Chemistry of Art > 5. Complexes > The Range of Colours That Can be Obtained From One Metal >
Process information from secondary sources to give an example of the range of colours that can be obtained from one metal such as Cr in different ion complexes
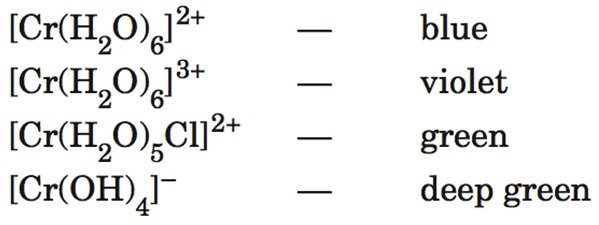
- The different colours of the complexes are due to the fact that energies of the d orbitals are effected by
- The metal ion
- Surrounding ligand groups
- These energy separations of the d orbital result in
- Differences to which frequencies of photons absorbed
- Hence differences to colour
- Complexes that have no d electrons or 10 d electrons cannot absorb visible light and hence are colourless
- Zn2+ and Sc3+
