[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]The Chemistry of Art > 5. Complexes > What Are Ligands Composed Of? >[/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Explain that ligands have at least one atom with a lone pair of electrons[/cs_text][cs_text]
- Co-ordinate covalent bonds form between the ligands and the transition metal ion
- Uses the lone pair electrons of the ligand
Lewis acids and bases:
- Lewis acids – Acceptor electron pair
- Lewis Bases – Donator electron pair
- Therefore, in complex ions
- Ligands are Lewis bases and metals ions are Lewis Acids
- The co-ordinate covalent bond formed is a Lewis acid- Lewis base interaction
- g Cu2+ reacting with ammonia
[/cs_text][cs_text]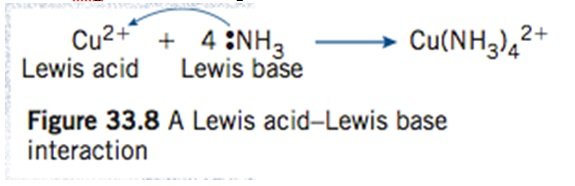 [/cs_text][cs_text]- Each NH3 donates a pair of electrons to form [Cu(NH3)4]2+[/cs_text][cs_text]
[/cs_text][cs_text]- Each NH3 donates a pair of electrons to form [Cu(NH3)4]2+[/cs_text][cs_text]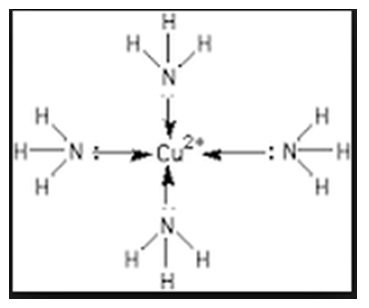 [/cs_text][cs_text]
[/cs_text][cs_text]
- Because all ligands are Lewis bases, they must have at least one pair of unshared electrons
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]