The Chemistry of Art > 5. Complexes > What Are Ligands Composed Of? >
Explain that ligands have at least one atom with a lone pair of electrons
- Co-ordinate covalent bonds form between the ligands and the transition metal ion
- Uses the lone pair electrons of the ligand
Lewis acids and bases:
- Lewis acids – Acceptor electron pair
- Lewis Bases – Donator electron pair
- Therefore, in complex ions
- Ligands are Lewis bases and metals ions are Lewis Acids
- The co-ordinate covalent bond formed is a Lewis acid- Lewis base interaction
- g Cu2+ reacting with ammonia
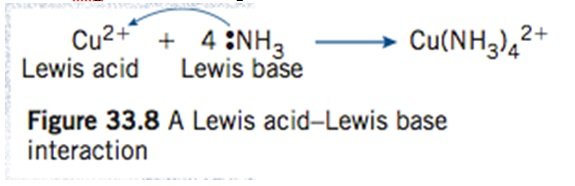
– Each NH3 donates a pair of electrons to form [Cu(NH3)4]2+
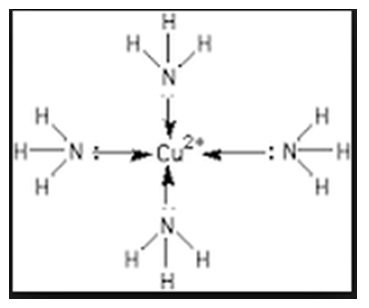
- Because all ligands are Lewis bases, they must have at least one pair of unshared electrons
