[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]The Chemistry of Art > 3. Electrons in the Atom > Electrons in Their Ground-State Electron Configurations >[/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Identify that electrons in their ground-state electron configurations occupy the lowest energy shells, subshells and orbitals available to them and explain why they are able to jump to higher energy levels when excited[/cs_text][cs_text]
- Electrons are placed in orbitals starting with the lowest energy orbital first
- The order in which orbitals fill depends upon their precise energy level
[/cs_text][cs_text]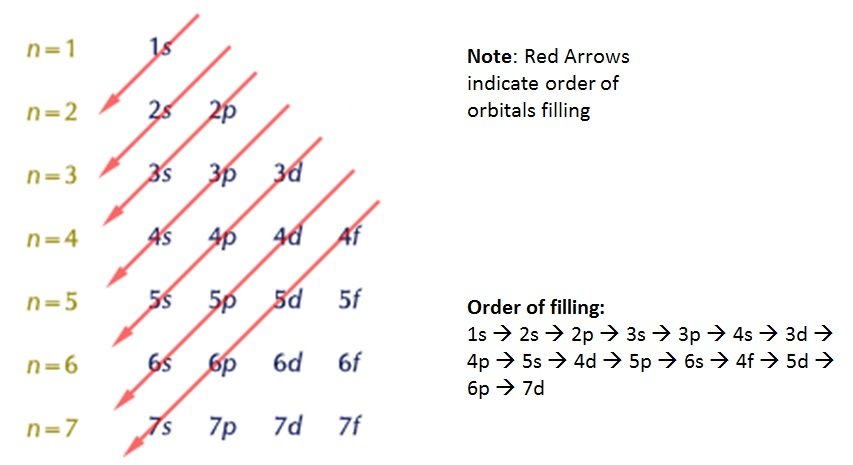 [/cs_text][cs_text]Example: Sodium
[/cs_text][cs_text]Example: Sodium
Na = 11 Electrons
- Ground state
- First two electrons in 1s orbital
- Next two located in the 2s orbital
- Next six located in the three orbitals of the 2p subshell
Eleventh electron located in the 3s subshell[/cs_text][cs_text]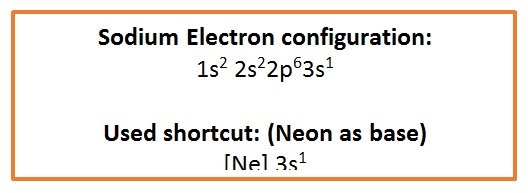 [/cs_text][cs_text]
[/cs_text][cs_text]
- Excited state
- Any state different from the ground state
[/cs_text][cs_text]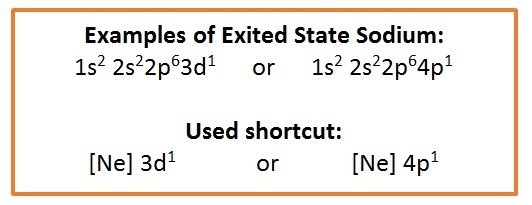 [/cs_text][cs_text]Movement of electrons across increasing energy levels:
[/cs_text][cs_text]Movement of electrons across increasing energy levels:
- Absorption of energy by an atom leads to change of electron configuration as a valence electron is promoted to a higher energy orbital
- Due to absorption of a precise quanta of energy (as above)
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]