[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]The Chemistry of Art > 3. Electrons in the Atom > Ionisation Energies > [/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]How trends in successive ionisation energies are used to predict the number of electrons in the outermost shell and the subshells occupied by these electrons[/cs_text][cs_text]Successive ionisation energies:
- It is possible to remove more than one electron from an atom
- Named in accordance with which electron is being removed
- e.g. Second ionisation is amount of energy for the second consecutive electron removal
- Removal order is the reverse of the order in which they are filled
- e.g. Oxygen (as above)
[/cs_text][cs_text]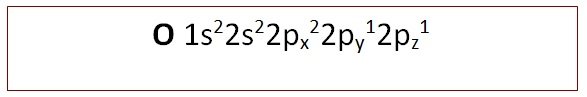 [/cs_text][cs_text]
[/cs_text][cs_text]
- Of the paired electrons from the 2p orbital is removed first
- The rest of the 2p electrons are removed one by one
- Then the 2s electrons
- Then the 1s electrons
- Successive ionization energies have larger values as
- Electrons are removed from progressively larger positive charges, hence requiring more energy to remove them
- Electrons in closer shells require more energy to remove due to proximity to the positive nucleus and less shielding from subsequent shells
- Exceptions
- If there are two electrons in one orbital, a relatively smaller amount of energy is needed due to electrostatic repulsion
The three facts above can be used to deduce the amount of electrons in the outermost shell and their subshells due to the resulting spikes in ionization energy[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]