The Chemistry of Art > 3. Electrons in the Atom > Using Hund’s Rule >
Process information from secondary sources to use Hund’s rule to predict the electron configuration of an element according to its position in the Periodic Table
- Hund’s rule states that the distribution of electrons in a free atom occur so that as many orbitals as possible are occupied by a single electron (spinning identically) before pairing of electrons with opposite spins occurs
- Three p orbitals
- Five d orbitals
- Seven f orbitlas
- Prediction examples: Seventh nitrogen
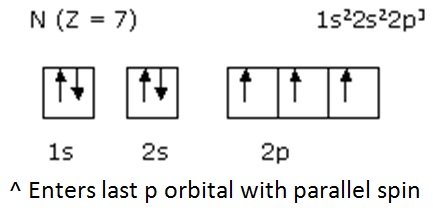
- Prediction examples: Eighth Oxygen
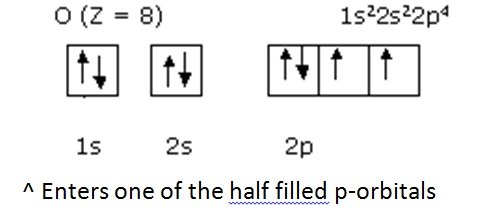
- Therefore has opposite spin
