[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]The Chemistry of Art > 4. The Transition Metals > Observing The Colour Changes of a Named Transition Element >[/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Perform a first-hand investigation to observe the colour changes of a named transition element as it changes in oxidation state[/cs_text][cs_text]Aim: To investigate the colour changes associated with changes in the oxidation state of manganese
Method:
- Specifics not needed
- Prepare a solution of ion with a high oxidation state and a low oxidation state
- Record their colours
- Make the ion with a high oxidation state reduce
- High Oxidiser
- Make the solution with a lower oxidation state oxidise
- High Reductor
- Record the colour changes in between as these cause further oxidation states to occur
[/cs_text][cs_text]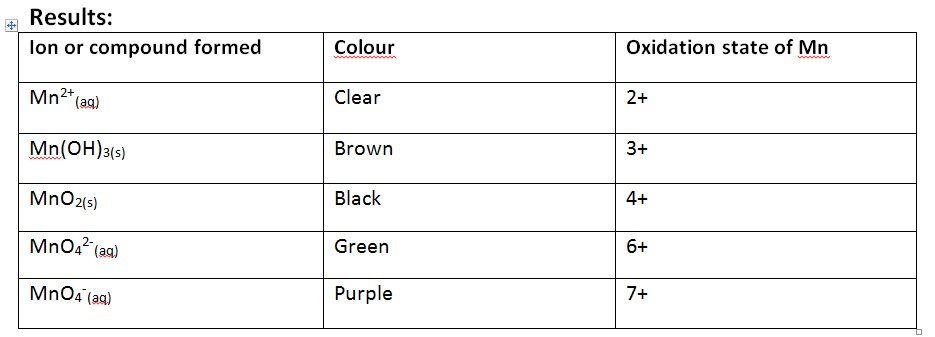 [/cs_text][cs_text]Accuracy:
[/cs_text][cs_text]Accuracy:
- Not largely accurate as the colours viewed are a qualitative measure and potentially subjective
Reliability:
- Repeated multiple times to ensure consistent results
Validity:
- Controlled
- Solutions have identical moles
- Identical amounts of solution added to achieve each oxidation state
- Answers aim
Variables:
Independent:
Oxidation state of Manganese
Dependent:
Colour displayed by each Manganese oxidation state[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]