The Chemistry of Art > 4. The Transition Metals > Observing The Colour Changes of a Named Transition Element >
Perform a first-hand investigation to observe the colour changes of a named transition element as it changes in oxidation state
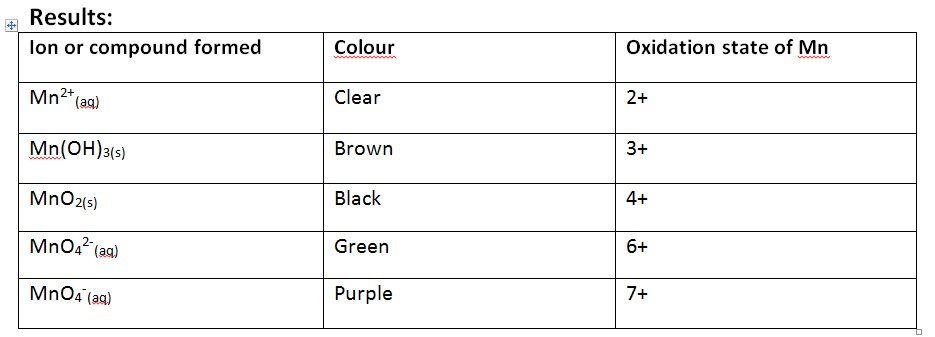
Aim: To investigate the colour changes associated with changes in the oxidation state of manganese
Method:
- Specifics not needed
- Prepare a solution of ion with a high oxidation state and a low oxidation state
- Record their colours
- Make the ion with a high oxidation state reduce
- High Oxidiser
- Make the solution with a lower oxidation state oxidise
- High Reductor
- Record the colour changes in between as these cause further oxidation states to occur
Accuracy:
- Not largely accurate as the colours viewed are a qualitative measure and potentially subjective
Reliability:
- Repeated multiple times to ensure consistent results
Validity:
- Controlled
- Solutions have identical moles
- Identical amounts of solution added to achieve each oxidation state
- Answers aim
Variables:
Independent:
Oxidation state of Manganese
Dependent:
Colour displayed by each Manganese oxidation state
