The Chemistry of Art > 4. The Transition Metals > What is a Transition Element? >
Define the term transition element
- Definition: Elements that form at least one ion with a partially filled sub-shell of d electrons
- Lesser definitions:
- Transition elements are those that have partially filled d-orbitals
- Transition elements are those that have properties intermediate between s-block and p-block elements
Elements in the d-block that are not transition metals:
- Scandium: Its only ion (Sc3+) has no electrons in the d sub-shell
Scandium atom:
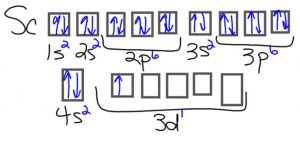

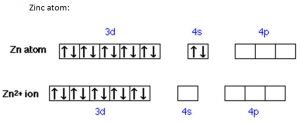
- Zinc: Its only ion (Zn2+) has a completely filled d sub-shell
Elements with unique orbital filling patterns: (For a later dotpoint but whatever)
- Filling typically occurs according to Hund’s rule
- Electrons in each of the 5 3d-orbitals
- 4s orbital already filled due to relative energy compared to 3p
- Exceptions to this rule are Chromium and Copper

- More stable when all d- orbital are half filled
- Electron moves from already filled 4s shell to accommodate

- More stable when all d-orbtials are filled with pairs
- Electron moves from already filled 4s shell to accommodate
