Forensic Chemistry > 6. All elements have identifiable emission spectra and this can be used to identify trace elements > Describing The Emission Spectrum of a Range of Elements >
Identify data, choose equipment, plan, and perform a first-hand investigation using flame tests and/or spectroscope analysis as appropriate to identify and gather first-hand information to describe the emission spectrum of a range of elements including Na and Hg
- Experiment: Atomic Emission Spectroscopy
- Safety:
- Wear safety glasses to avoid corrosive chemicals from spray getting into eye
- Avoid contact with the power source of spectral tubes – x-ray emission could cause tissue damage
- Wear protective gear, such as gloves, mask and eye goggles, when preparing heaavy metal solutions such as mercury, zinc, lead, and arsenic.
- Results:
- Since metal ions are soluble in chlorides or nitrates, all metals tested were chlorides or nitrates in solution
- Mercury and sodium are heavy metals, so the ions are volatile and can accumulate in human tissue. They are therefore observed in a spectral tube
- The predominant colour observed for mercury was blue/purple
- The sodium was a bright yellow
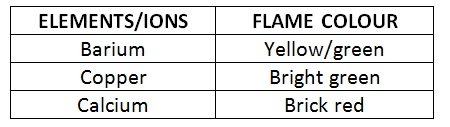
- Conclusion:
- When excited by flame, electrons emit waves of characteristic wavelengths and colors. Therefore, the characteristic color produced in a flame test can be used to identify unknown metal ions.
