Industrial Chemistry > 6. The Solvay process has been in use since the 1860s > The Steps Used in The Solvay Process >
Identify, given a flow chart, the sequence of steps used in the Solvay process and describe the chemistry involved in: brine purification, hydrogen carbonate formation, formation of sodium carbonate, ammonia recovery
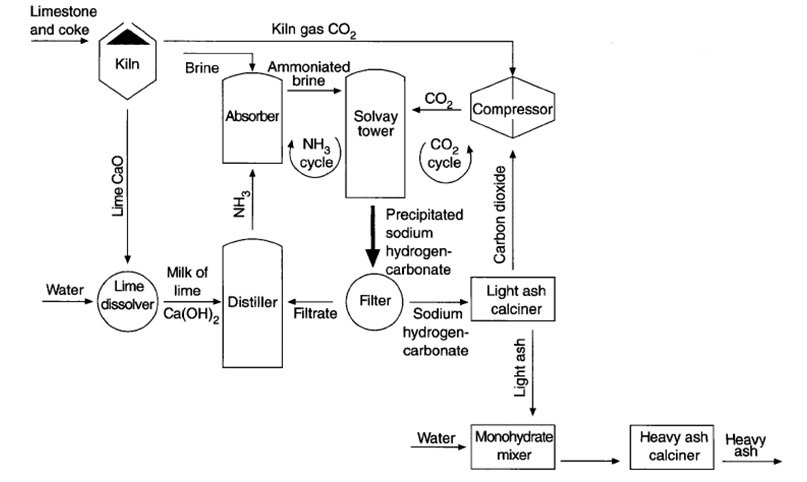
Shown above is a simplified flow diagram for the Solvay process. Each step is analysed below.
- STEP 1: Brine purification + addition of ammonia
Brine is mostly found in the ocean’s salt water and it may contain many impurities, such as Ca and Mg ions, which are undesirable in the Solvay process. The purification of brine was done by removing those impurities and concentrating the sodium chloride solution. The removal of those impurities can be done via two methods.
- Precipitation of the Ca and Mg ions by reacting them with either sodium carbonate or sodium hydroxide.
Ca2+ (aq) + CO32- à CaCO3 (s)
Mg2+ (aq) + 2 OH– (aq) à Mg(OH)2 (s)
- Fractional crystallisation – where the more soluble contaminants are crystallised out from the brine solution.
The purified brine will now be introduced to ammonia saturator. Here, the brine flows down with ammonia over many partitions of the said vessel. The solution will then be cooled using ice-cooled water resulting to the formation of ammoniated brine.
- STEP 2: Production of sodium hydrogen carbonate
The ammoniated brine will then be passed into the Solvay tower or commonly known as carbonator. The ammoniated brine, which consists ammonia, water and sodium chloride, will react with carbon dioxide. The carbon dioxide came from the decomposition of calcium carbonate (limestone) at high temperature.
CaCO3 (s) à CaO (s) + CO2 (g)
HEAT
The carbon dioxide reacts with water to produce carbonic acid.
CO2 (g) + H2O (l)↔ H2CO3 (aq)
Carbonic acid, which is a weak acid, will then neutralize the ammonia, which is a weak base. The neutralization reaction produces ammonium and hydrogen carbonate ions.
H2CO3 (aq) + NH3 (aq) ↔ HCO3– (aq) + NH4+ (aq)
These hydrogen carbonate ions will then react with sodium ions (from the NaCl) to produce sodium hydrogen carbonate (NaHCO3).
HCO3– (aq) + Na+ (aq)↔ NaHCO3 (s)
The reaction mixture is then cooled to about 0ºC which allows the aqueous sodium hydrogen carbonate to crystallize out leaving ammonium chloride in the solution. The overall reaction can be described as:
NH3 (g) + NaCl (aq) + H2O (l) + CO2 (g) à NH4Cl (aq) + NaHCO3 (s)
- STEP 3: Formation of sodium carbonate
The mixture of the solid NaHCO3 and aqueous NH4Cl will then be filtered to collect the desired solids. The collected NaHCO3 will then be heated in a furnace to about 300ºC. This would result to the formation of sodium carbonate, water and carbon dioxide. The carbon dioxide is recycled back into the carbonator for the production of NaHCO3. Recycling CO2 would reduce the cost.
- 2 NaHCO
3 (s)
- à Na
2
- CO
3 (s)
- + CO
2 (g)
- + H
2
- O
(l)
- HEAT
- STEP 4: Recovery of ammonia
The calcium oxide (lime) from the decomposition of the CaCO3 (Step 2) will be reacted with water to form calcium hydroxide (slaked lime). This is shown in the equation below:
CaO (s) + H2O (l) à Ca(OH)2 (aq)
The obtained calcium hydroxide will then be used to react with ammonium chloride. Ammonium chloride is present in the filtrate from the hydrogen carbonate formation process, which was also produced in step 2.
Ca(OH)2 (s) + 2 NH4Cl (aq) à CaCl2 (aq) + 2 NH3 (g) + 2 H2O (l)
The ammonia is very expensive to be manufactured, thus, the recovered one is being recycled. Lastly, CaCl2 is also produced as a main by-product.
