[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]The Chemistry of Art > 2. The Structure of the Atom and Colours > Explaining The Flame Colour >[/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Explain the flame colour in terms of electrons releasing energy as they move to a lower energy level [/cs_text][cs_text]Light:
- Light or electromagnetic (EM) radiation both waves and particulate properties, have different types such as, gamma rays, x-rays, ultra-violet (UV) rays, visible light, infra-red (IR), microwaves and radiowaves (TV/ FM/AM)\
- When light strikes an object, it can be absorbed, reflected or only particular wavelengths is absorbed and the rest is transmitted/reflected.
- Waves are characterized by:
- Wavelength: distance between two successive waves
- Frequency: number of cycles per second that pass a given point in time
v = c/Δ
where v – frequency (in Hertz or s-1)
Δ – wavelength (in meter)
c – speed of light (3 x 108 m/s)
- Human eye is sensitive to the visible region of the EM spectrum
- Beyond red end = Infra-Red region
- Beyond Blue end = Ultraviolet region
- Different frequencies of visible light = different wavelengths of visible light = varying observed colours
- Frequency (v) is directly proportional to energy of photon (E), however inversely proportional to wavelength (λ)
E = hv = h c/Δ
- High frequency waves = High energy of Photon= Low wavelength
- Light given off by energetically excited atom is not a continuous distribution of wavelength
- Each element has its own unique spectral signature
Flame Colour of Elements:
- Flame colour is produced when a granule of ionic compound or a drop of its solution are placed in a flame
- Some of the commonly known metallic elements have known to strongly emit light in the visible region of EM spectrum
- Through absorbing or emitting discrete amount of energy (quantized energy called photons), electrons are able to move to different orbits
- Once an electron becomes excited (electron in higher energy level), photons are released after it reached the ground state (electron in original energy level ) resulting to the formation of spectral line (wavelength of light)
- The wavelength which is associated to the observed colour is dependent on the change in energy level and orbit of the electron
- The colour exhibited/reflected by an object is explained as that:
- All wavelengths of white light that strikes through a substance are absorbed except for a particular wavelength which is consequently reflected
Example: For a given material, all wavelengths of visible spectrum are absorbed except for yellow wavelength (reflected) upon contact with white light, thus yellow colour is observed.
- When a substance absorbs a particular wavelength from white light that passed through, the complementary wavelength to the absorbed specific wavelength is reflected or observed
Example: Blue absorbed = Orange observed[/cs_text][cs_text]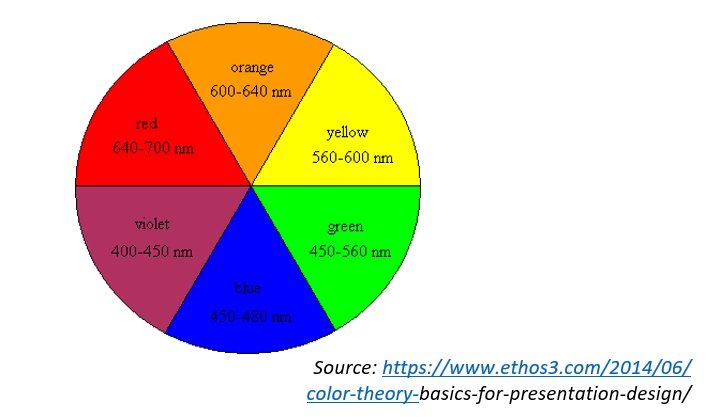 [/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]