[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]Forensic Chemistry > 5. Much forensic evidence consists of very small samples and sensitive analytical techniques are required > Techniques in Analyzing Small Samples > [/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Identify, outline and assess the value of the following techniques in the analysis of small samples[/cs_text][cs_text]
- In all chromatographic separations, the sample is dissolved in a mobile phase, which may be a gas or a liquid. This mobile phase is then forced through an immiscible stationary phase, which is fixed in place in a column or on a solid surface.
- The two phases are chosen so that the components of the sample distribute themselves between the mobile and stationary phases to varying degrees.
- Those components strongly retained by the stationary phase moves slowly with the flow of the mobile phase. In contrast, components that are weakly held by the stationary phase travels faster.
- A chromatogram shows a series of peaks dependent on the retention time of the different components (samples with longer retention times appear later). The peak area is a measure of the concentration of each species in the mixture. However, peak height can be used as a substitute for peak area if the peaks observed are narrow. The chromatogram is calibrated with known standards so that these components can be identified. The presence of a peak at the same retention time as control compound verifies the presence of that component.
Gas-liquid chromatography
- Non-destructive form of testing which separates substances on the basis of their distribution in the stationary phase (porous solid)
- e.g. used in urine samples to detect blood alcohol levels
[/cs_text][cs_text]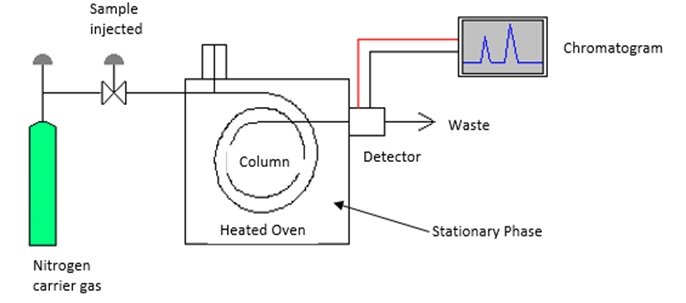 [/cs_text][cs_text]
[/cs_text][cs_text]
- In the stationary phase, the sample is vaporised & injected into a column containing a specific solid such as carbon or silica. In the mobile phase, the different components in the sample pass through a column with an inert carrier gas (such as nitrogen, helium, argon or carbon dioxide) at different rates and are detected as they emerge from the column. Towards the end of the column is a detector which signals when a component is leaving the column.
- Advantages:
- Requires a small amount of sample with little preparation
- High sensitivity to volatile organic mixtures at concentrations in parts per billion range
- Very effective, relatively quick and easily performed
- Disadvantages:
- Results are not an absolute mean of identification as different compounds can have the same retention time
- Slow vaporization of the stationary phase leads to change in column criteria, thereby contaminating the sample
- Expensive
High performance liquid chromatography
- Separation is due to the different solubilities of the components in the mobile and stationary phases
- Two types of separation can be utilized in HPLC depending on the polarity of the solvent and the stationary phase:
- Normal phase liquid chromatography: uses columns packed with polar stationary phases combined with non-polar or moderately-polar mobile phases to separate the components of mixtures
- Reversed phase liquid chromatography: Separation based on hydrophobicity under conditions where the chromatography medium is more hydrophobic than the mobile phase
- The solution is pumped into the column where a high pressure pump pushes the solvent and the mobile phase into the column. The high pressure is necessary to overcome the resistance of the tiny particles in the sample. The components are separated depending on its polarity with the stationary and the mobile phases. The separated components are then detected and the detector feeds the signal to the read-out system in order to generate the chromatogram.
[/cs_text][cs_text]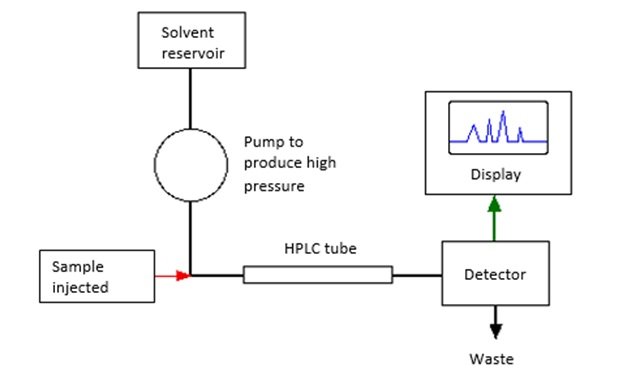 [/cs_text][cs_text]
[/cs_text][cs_text]
- Key considerations on the separation process:
- If the component has high solubility in the mobile phase but has low solubility in the stationary phase, it will move through the column fastest and will appear in the chromatogram first.
- If the component has low solubility in the mobile phase but has high solubility in the stationary phase, it will move through the column slowest and will appear in the chromatogram last.
- Advantages:
- Versatile and precise in identifying and quantifying chemical compounds
- Can be performed at room temperature which makes it capable to separate and to analyse heat-sensitive compounds without degrading them
- Very sensitive – can detect nanogram quantities
- Has a high resolution
- Disadvantages:
- Can be costly requiring large amounts of expensive compounds
- Low sensitivity for some compounds
- Irreversibly adsorbed compounds are not detected
- Columns are expensive and easily degradable
- Since samples are small, care must be taken to avoid contamination
- Machine must be calibrated with known standards
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]