[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]Forensic Chemistry > 6. All elements have identifiable emission spectra and this can be used to identify trace elements > Describing The Emission Spectrum of a Range of Elements >[/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Identify data, choose equipment, plan, and perform a first-hand investigation using flame tests and/or spectroscope analysis as appropriate to identify and gather first-hand information to describe the emission spectrum of a range of elements including Na and Hg[/cs_text][cs_text]
- Experiment: Atomic Emission Spectroscopy
- Safety:
- Wear safety glasses to avoid corrosive chemicals from spray getting into eye
- Avoid contact with the power source of spectral tubes – x-ray emission could cause tissue damage
- Wear protective gear, such as gloves, mask and eye goggles, when preparing heaavy metal solutions such as mercury, zinc, lead, and arsenic.
- Results:
- Since metal ions are soluble in chlorides or nitrates, all metals tested were chlorides or nitrates in solution
- Mercury and sodium are heavy metals, so the ions are volatile and can accumulate in human tissue. They are therefore observed in a spectral tube
- The predominant colour observed for mercury was blue/purple
- The sodium was a bright yellow
[/cs_text][cs_text]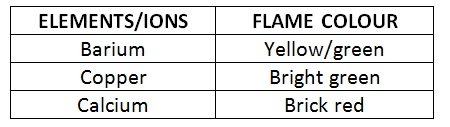 [/cs_text][cs_text]
[/cs_text][cs_text]
- Conclusion:
- When excited by flame, electrons emit waves of characteristic wavelengths and colors. Therefore, the characteristic color produced in a flame test can be used to identify unknown metal ions.
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]