[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]Industrial Chemistry > 2. Many industrial processes involve manipulation of equilibrium reactions > Process And Present Information From Secondary Sources to Calculate K From Equilibrium Conditions >[/cs_text][cs_text style=”color: #800000;”]Process and present information from secondary sources to calculate K from equilibrium conditions[/cs_text][cs_text]A reaction vessel contains NH3, N2, and H2 at equilibrium at a certain temperature. The equilibrium concentrations are [NH3] = 0.25 M, [N2] = 0.11 M, and [H2] = 1.91 M. Calculate the equilibrium constant K for the synthesis of ammonia if the reaction is represented as
(a) N2(g) + 3H2(g) ⇌ 2NH3(g)
(b) ½ N2(g) + 3/2 H2(g) ⇌ NH3(g)
SOLUTION:
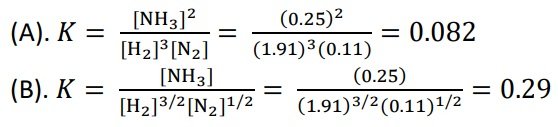
A 2.50-mole quantity of NOCl was initially in a 1.50-L reaction chamber at 400°C. After equilibrium was established, it was found that 28.0 percent of the NOCl had dissociated:
2NOCl(g) ⇌ 2NO(g) + Cl2(g)
Calculate the equilibrium constant Kc for the reaction.
SOLUTION:

For NOCl, there is a 28% decrease in the initial concentration.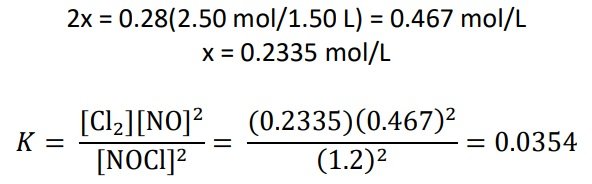 [/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]