[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]Industrial Chemistry > 5. Saponification is an important organic industrial process > The Cleaning Action Of Soap >[/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Account for the cleaning action of soap by describing its structure[/cs_text][cs_text] [/cs_text][cs_text]
[/cs_text][cs_text]
- Hydrocarbon tail or hydrophobic tail – is water-hating zigzag line above that is non polar in nature
- Hydrophilic head – is the water-loving anion shown as negative circle above which is polar in nature
Cleaning Action of Soap:
- The non-polar dirt, oil droplet for example, forms intermolecular interaction with the non-polar portion or hydrophobic tail of the soap molecules, making the long hydrocarbon tails be embedded in the oil molecule.
Rule: “like dissolves like” – polar interacts with polar while non-polar interacts with non-polar[/cs_text][cs_text]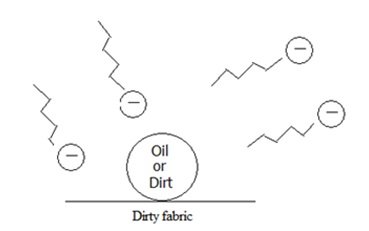 [/cs_text][cs_text]
[/cs_text][cs_text]
- Water molecules are repelled by the non-polar hydrocarbon tail since water is polar, however are retained due to the hydrophilic head (water-loving) part of the soap which forms hydrogen bonding with water molecules.
- The intermolecular attraction between the long hydrocarbon tails with the oil droplet and the hydrophilic head with the water molecules are strong enough to remove the oil droplet from fabric, leaving the oil droplet (micelles) suspended in the water.
[/cs_text][cs_text]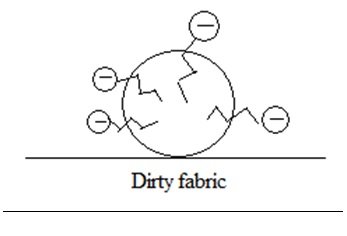 [/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]