[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]Shipwrecks and Salvage > 3. Electrolytic Cells >
Describe, using half-equations, what happens at the anode and cathode during electrolysis of selected aqueous solutions[/cs_text][/cs_column][/cs_row][/cs_section][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]
- Electrolysis: The passage of current through an electrolyte, causing chemical change at the electrodes.
- In selecting the appropriate half-equations to describe the reaction occurring at each electrode in electrolytic reactions, the following guides should be considered:
- The higher the reduction potential, the harder it is to oxidise the anion.
- Before water molecules are oxidised to oxygen, bromine ions and iodine ions are oxidised.
- Nitrate ions, sulfate ions and fluoride ions are never oxidised, with water being oxidised to oxygen instead.
- The lower the electrode potential, the harder it is to reduce a metal ion.
- Silver ions and copper ions are always reduced.
- Potassium ions, sodium ions, calcium ions and aluminum ions are never reduced.
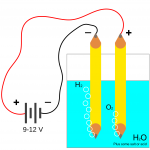
- In electrolysis reactions:
- Reduction occurs at the cathode, or negative electrode.
- Oxidation occurs at the anode, or positive electrode.
- The charge on the electrodes is different for an electrolytic cell and a galvanic cell:
- The cathode is the negative in an electrolytic cell.
- The cathode is the positive in a galvanic cell.
- Anions carry charge towards the anode.
- Cations carry charge towards the cathode.
- The reactions that occur at the anode and the cathode depend on the voltage.
 [/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]