[cs_content][cs_section parallax=”false” separator_top_type=”none” separator_top_height=”50px” separator_top_angle_point=”50″ separator_bottom_type=”none” separator_bottom_height=”50px” separator_bottom_angle_point=”50″ style=”margin: 0px;padding: 45px 0px;”][cs_row inner_container=”true” marginless_columns=”false” style=”margin: 0px auto;padding: 0px;”][cs_column fade=”false” fade_animation=”in” fade_animation_offset=”45px” fade_duration=”750″ type=”1/1″ style=”padding: 0px;”][cs_text]The Chemistry of Art > 5. Complexes > The Range of Colours That Can be Obtained From One Metal >[/cs_text][cs_text style=”color: #800000;font-family: “Oxygen”,sans-serif;”]Process information from secondary sources to give an example of the range of colours that can be obtained from one metal such as Cr in different ion complexes[/cs_text][cs_text]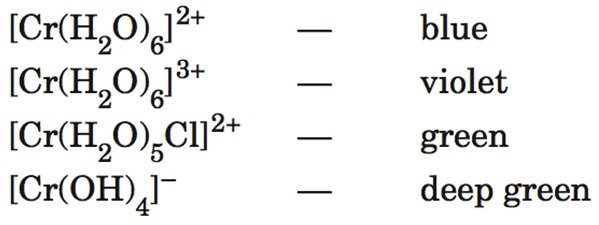 [/cs_text][cs_text]
[/cs_text][cs_text]
- The different colours of the complexes are due to the fact that energies of the d orbitals are effected by
- The metal ion
- Surrounding ligand groups
- These energy separations of the d orbital result in
- Differences to which frequencies of photons absorbed
- Hence differences to colour
- Complexes that have no d electrons or 10 d electrons cannot absorb visible light and hence are colourless
- Zn2+ and Sc3+
[/cs_text][/cs_column][/cs_row][/cs_section][/cs_content]